1 to 30 elements atomic mass
The atomic mass of an element is the average mass of its atoms measured in atomic mass units amu, commonly known as daltons, D.
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next. What is Scribd?
1 to 30 elements atomic mass
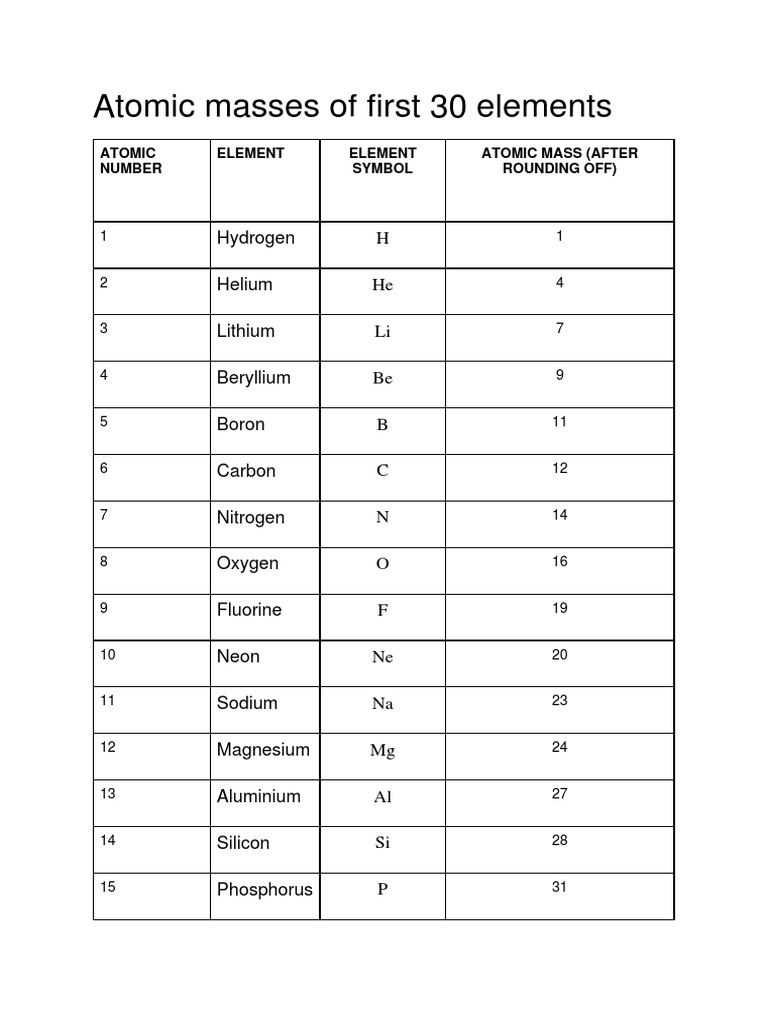
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass. There are many ways to find the atomic mass of an element, but the easiest way is to look it up on the periodic table of elements. Put your understanding of this concept to test by answering a few MCQs. One Mole of a substance is defined as the total number of atoms in 12 grams of Carbon isotope. Atomic mass is the average mass of the protons, neutrons, and electrons in an atom. An Atomic mass unit or one amu is the mass unit equivalent to the one-twelfth mass of one atom of the carbon isotope. Carbon was chosen as a reference element for calculating atomic mass because it is naturally occurring and is present in abundance. The atomic mass unit is the full form of amu. It is equivalent to the one-twelfth mass of one atom of the carbon isotope.
It is expressed as a decimal fraction.
Buka menu navigasi. Tutup saran Cari Cari. Pengaturan Pengguna. Lewati carousel. Karusel Sebelumnya. Karusel Berikutnya. Apa itu Scribd?
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass.
1 to 30 elements atomic mass
Even though atoms are very tiny pieces of matter, they have mass. Their masses are so small, however, that chemists often use a unit other than grams to express them—the atomic mass unit. Masses of other atoms are expressed with respect to the atomic mass unit. For example, the mass of an atom of 1 H is 1. Note, however, that these masses are for particular isotopes of each element. How, then, do we describe the mass of a given element?
Adapter macbook air hdmi
What is the Atomic Mass of Chlorine? Periodic Law Periodic Law. Isotopes are atoms of the same element that have different numbers of neutrons. Thank you for your valuable feedback! What is the Atomic Mass of Fluorine? Why Isotopes have different Physical Properties? Carbon, for example, is a typical carbon atom with six neutrons and six protons. Adenike nikky November 27, at am. The mass a molecule carries is known as its molecular mass, additionally known as molecular weight. These are the first 30 elements in the periodic table, listed in order of increasing atomic number. In this article, we will learn about the Atomic Mass of Elements 1 to 30 with Symbols. This was the complete discussion on atomic mass, its calculation and the difference between atomic mass and number. Message Message. Arya August 8, at pm.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
It refers to the average weight of a particular element. Informasi Dokumen klik untuk memperluas informasi dokumen This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements. Close suggestions Search Search. Is this content inappropriate? An element which has the same atomic number can have multiple isotopes with different numbers of neutrons. The atomic mass formula is shown below. Uncharged subatomic particles known as neutrons are stable when contained in an atomic nucleus. The atomic mass is determined by averaging the weight of all the isotopes of the element. What are Divalent Ions? Gram Atomic and Gram Molecular Mass.

Excuse for that I interfere � I understand this question. It is possible to discuss. Write here or in PM.
In it something is also to me it seems it is good idea. I agree with you.
It is remarkable, very much the helpful information