2agcl
Silver chloride is an 2agcl chemical compound 2agcl the chemical formula Ag Cl, 2agcl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts to silver and chlorine2agcl, which is signaled by grey to black or purplish coloration in some samples.
Then, lookup atomic weights for each element in periodic table : Ba: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
2agcl
.
Periodic table. Gas laws.
.
Well you gots the stoichiometric equation And silver chloride is as soluble as a BRICK, and precipitates from aqueous solution as a curdy white solid. When you try to collect the silver chloride it is a very curdy, unhandy precipitate. And yet, at one stage, given the photosensitivity of the silver chloride which sometimes you can see , plates of silver chloride were once used as the basis for black and white photography. But back to the question How much barium chloride is necessary to react with the silver nitrate in last question? Mar 16, Explanation: And silver chloride is as soluble as a BRICK, and precipitates from aqueous solution as a curdy white solid. And so we interrogate the molar quantities Related questions How do I determine the molecular shape of a molecule?
2agcl
Then, lookup atomic weights for each element in periodic table : Ag: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Bulldozer sneakers
CaCl CaCl 2. TlCl TlCl 3. Chemistry of Arsenic, Antimony and Bismuth. Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. In , Schulze made a paste of silver nitrate and chalk, placed the mixture in a glass bottle, and wrapped the bottle in PtCl 2 PtCl 4. Retrieved 19 June Encyclopedia of Physical Science and Technology Third ed. Upon illumination or heating, silver chloride converts to silver and chlorine , which is signaled by grey to black or purplish coloration in some samples. Archived from the original PDF on December 3, Above 7. The history of the discovery of photography. Chemical forum. Other names Cerargyrite Chlorargyrite Horn silver Argentous chloride. Chemistry and Technology of Printing and Imaging Systems.
Silver chloride is a white crystalline chemical compound with the formula AgCl.
It is easily synthesized by metathesis : combining an aqueous solution of silver nitrate which is soluble with a soluble chloride salt, such as sodium chloride which is used industrially as a method of producing AgCl , or cobalt II chloride. Toggle limited content width. Retrieved 19 June Common compound names. Retrieved Chemical compound. Cyanidation produces the soluble dicyanoargentate complex, which is later turned back to silver by reduction. Interfering ions for this test are bromide and iodide, as well as a variety of ligands see silver halide. PbCl 2 PbCl 4. Read Edit View history. Download as PDF Printable version. Cerargyrite Chlorargyrite Horn silver Argentous chloride. N verify what is Y N? Retrieved 20 June

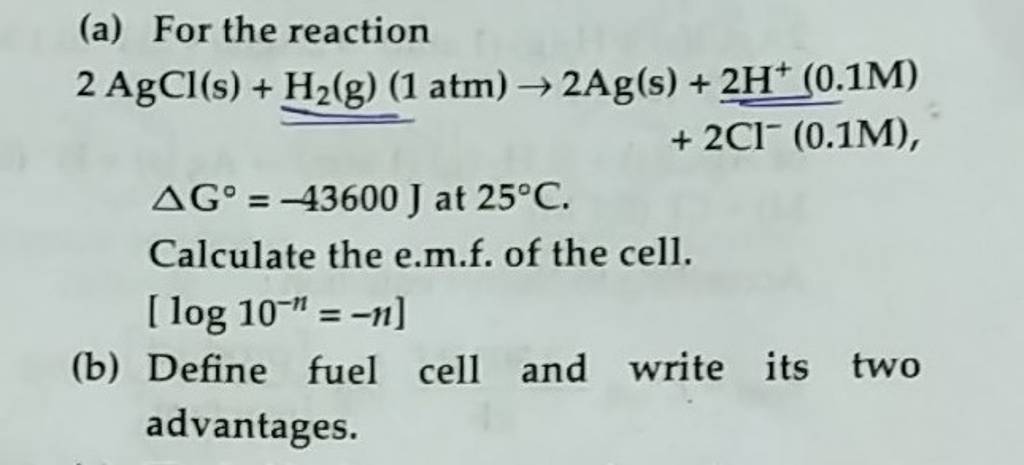
In my opinion, you are mistaken.
I consider, that you are not right. I can prove it. Write to me in PM, we will discuss.