Acetic acid lewis dot structure
Weekly Web Work 7: a. Submissions are no longer accepted.
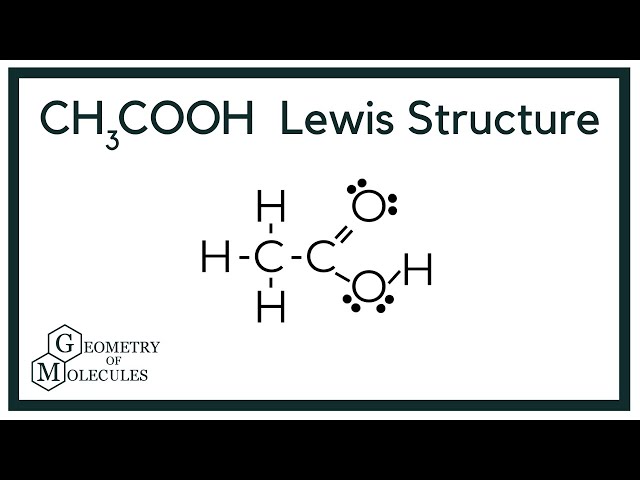
Lewis Dot Structures. This assignment is past due and can no longer be submitted. The purpose of this assignment is to practice drawing Lewis dot structures. You will need to have a pencil and paper to use while working on this assignment. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons. Carbon has 4, oxygen has 6, and hydrogen has 1 valence electron. So, two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons.
Acetic acid lewis dot structure
.
The purpose of this assignment is to practice drawing Lewis dot structures. Exam Info. If not, you need to add lone pairs to satisfy each atom.
.
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. But as per the rule, we have to keep hydrogen outside.
Acetic acid lewis dot structure
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens. Connect 3 hydrogen atoms to the carbon atom with a single bond and another to one oxygen.
Melonsclips
Why could this be? Weekly Web Work 7: a. If not, do what all chemists do and rework your structure. Keys to Success. Please type your last name, first name:. Remember each dash represents two electrons and each lone pair is two electrons. How many valence electrons does chloroform have? How many single bonds does chloroform have? Does the total number of electrons in your Lewis structure match the number of valence electrons actually available? First, determine the number of valence electrons.
We begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction between two identical neutral atoms—for example, the H 2 molecule, which contains a purely covalent bond. Each hydrogen atom in H 2 contains one electron and one proton, with the electron attracted to the proton by electrostatic forces.
Examine your initial attempt to draw the Lewis structure of chloroform. The purpose of this assignment is to practice drawing Lewis dot structures. Absence Excuse Form. How is your structure for CS 2 similar to your structure for CO 2? Let's draw a Lewis structure for chloroform. Does every atom have an octet? Our Chemical World. You will need to have a pencil and paper to use while working on this assignment. Keys to Success. You may change your mind as often as you wish. Submissions are no longer accepted. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons. Carbon has 4, oxygen has 6, and hydrogen has 1 valence electron. Let's draw a Lewis structure for chloroform. So, two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons.

I suggest you to come on a site on which there is a lot of information on this question.
Excuse, that I interfere, but I suggest to go another by.
You were visited with simply brilliant idea