Aluminium sulfate ionic formula
Al 2 SO 4 3 is a chemical compound with the chemical name Aluminium sulphate. Aluminium sulphate is aluminium sulfate ionic formula called Filter Alum or Dialuminum trisulphate. It is a white crystalline solid in its anhydrous form and in its solution form it appears as a colourless liquid. Both the forms are non-toxic and non-combustible.
Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. This is an Inorganic salt that is made from the neutralization reaction of Aluminium Hydroxide and sulfuric acid. It is an edible substance used for drinking water purification, as a pickling agent, and as one of the components in baking powder. Aluminium sulfate is also used as a chemical that enhances the immune response. It reduces the growth of bacteria on the skin. Aluminium sulfate is also used as a preservative.
Aluminium sulfate ionic formula
.
Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. Aluminium sulfate is a very good Coagulant which makes it used as a Waterproof agent in construction work.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula.
Aluminium sulfate ionic formula
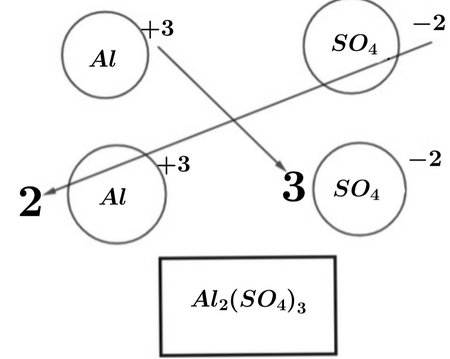
Aluminium sulfate is a salt with the formula Al 2 SO 4 3. It is soluble in water and is mainly used as a coagulating agent promoting particle collision by neutralizing charge in the purification of drinking water [3] [4] and wastewater treatment plants , and also in paper manufacturing. The anhydrous form occurs naturally as a rare mineral millosevichite , found for example in volcanic environments and on burning coal-mining waste dumps. Aluminium sulfate is rarely, if ever, encountered as the anhydrous salt. Aluminium sulfate is sometimes called alum or papermaker's alum in certain industries. The alum schists employed in the manufacture of aluminium sulfate are mixtures of iron pyrite , aluminium silicate and various bituminous substances, and are found in upper Bavaria , Bohemia , Belgium , and Scotland. These are either roasted or exposed to the weathering action of the air.
Fhm helen flanagan
It is also utilized in styptic pencils, which helps to stop the bleeding during minor cuts. Your Mobile number and Email id will not be published. The ionic bond is formed between Aluminium and Oxygens from Sulfate ions. Side Effects of Aluminium Sulfate Although Alum is an edible and non-toxic chemical compound, using it in excess and neglecting manner may lead to some toxic results such as Irritations and burning on the skin. Along with the disease-causing microorganisms, unrelated chemicals called Adjuvants are also added to the vaccines to enhance the response of the Immune system. Aluminium sulphate is a chemical compound produced with Al2 SO4 3. How dangerous is aluminium sulphate? Here, when acid is reacting with the basic Aluminium hydroxide salt and water is created as a product. Your result is as below. In construction, works as a waterproofing agent Aluminium sulfate is a very good Coagulant which makes it used as a Waterproof agent in construction work. Download Now. View Test Series.
Explore the properties, applications, potential hazards, and environmental implications of aluminum sulfate in this comprehensive guide.
But when it is reacting with a strong acid, it serves as a weak base. What Is Silicon. This reaction is known to be a Double Displacement reaction. This has an acidic flavour. FREE Signup. In Sewage treatment Aluminium sulfate is an inorganic salt that is widely used in wastewater treatment as a Coagulant. The pH of Aluminium sulfate is between 5. Aluminium Sulfate Aluminium sulfate is a white-colored crystalline salt formed by the reaction of an acid sulfuric acid and Base Aluminium hydroxide. Share Share Share Call Us. Preparation of Aluminium Sulfate There are different methods involved in the preparation of Aluminium Sulfate. In the manufacture of paper Aluminium sulfate is used as a component in the manufacture of paper as it changes the absorbing properties of the paper. In a sulfate molecule, each sulfur is surrounded by four oxygen atoms. Gastrointestinal bleeding due to ingestion. Test Series.

Unfortunately, I can help nothing. I think, you will find the correct decision. Do not despair.
I am very grateful to you. Many thanks.