Ammonium formula
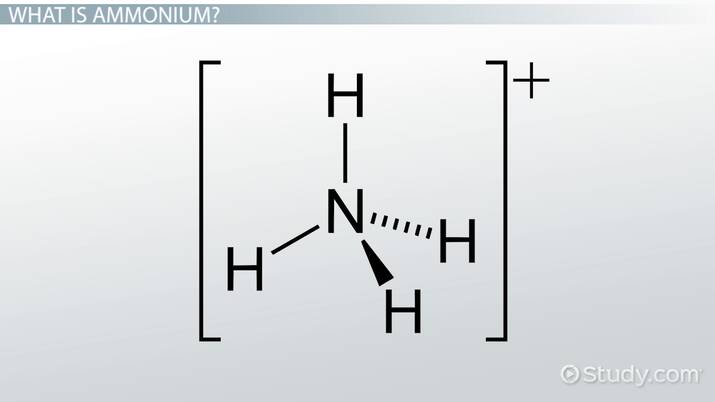
A cation is an electron-deficient species that carries a positive charge, ammonium formula. Ammonium formula polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero, ammonium formula. In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, and 1 lone pair of electrons complete the valence shell configuration. It is an electron-rich species nucleophile because it has one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile.
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula N H 3. A stable binary hydride and the simplest pnictogen hydride , ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste , and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products.
Ammonium formula
Molfile expand. Any bacterial metabolite produced during a metabolic reaction in Escherichia coli. Any fungal metabolite produced during a metabolic reaction in Baker's yeast Saccharomyces cerevisiae. Any mammalian metabolite produced during a metabolic reaction in humans Homo sapiens. An organic molecule or ion usually a metal ion that is required by an enzyme for its activity. It may be attached either loosely coenzyme or tightly prosthetic group. Read more News Our impact Contact us Intranet. Privacy Notice and Terms of Use. ChEBI Ontology. Automatic Xrefs. ChEBI Name.
Chemical Energy. Journal of the American Chemical Society.
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice. Each ion is surrounded by ions of opposite charge.
Ammonium formula
Ammonia and ammonium are nitrogen -containing compounds. Both are polyatomic compounds that are composed of more than two atoms per molecule or ion. Ammonium is derived from ammonia. Ammonia can be found as a gas while Ammonium can be found in all three phases of matter. Though they have nearly similar chemical compositions, there are many differences between ammonia and ammonium. The main difference between ammonia and ammonium is that ammonia is a neutral compound whereas ammonium is a cation. Ammonia is an inorganic compound having the chemical formula NH 3. It is a gaseous compound. The molar mass of ammonia is This compound is alkaline and has a characteristic pungent odor.
Dc comics kate kane
Bureau of Ships ] maximum allowable concentrations MACs : for continuous exposure 60 days is 25 ppm; for exposure of 1 hour is ppm. Other organisations have varying exposure levels. London: Charles Griffin and Company. Within a cell, glutamate serves this role. Struvite magnesium ammonium phosphate is thereby precipitated, and the yield of struvite can be increased by then treating the solution with bittern , a magnesium-rich solution that is a byproduct of making salt from sea water. Compared to hydrogen as a fuel , ammonia is much more energy efficient, and could be produced, stored and delivered at a much lower cost than hydrogen, which must be kept compressed or as a cryogenic liquid. Its energy density by volume is nearly double that of liquid hydrogen. Anhydrous ammonia corrodes copper - and zinc -containing alloys , which makes brass fittings not appropriate for handling the gas. Inorganic Chemistry. Listed below is a sample of journal articles that highlights the range of detectors that have been used to identify ammonia.
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:.
Saccharomyces cerevisiae NCBI:txid Other organonitrogen compounds incllude alprazolam , ethanolamine , ethyl carbamate and hexamethylenetetramine. OSTI Bureau of Ships ] maximum allowable concentrations MACs : for continuous exposure 60 days is 25 ppm; for exposure of 1 hour is ppm. Clemmensen Reduction. Before the availability of natural gas, hydrogen as a precursor to ammonia production was produced via the electrolysis of water or using the chloralkali process. Retrieved 2 February NGC and M 82". International Journal of Food Microbiology. Archived from the original on 23 August Related compounds.

0 thoughts on “Ammonium formula”