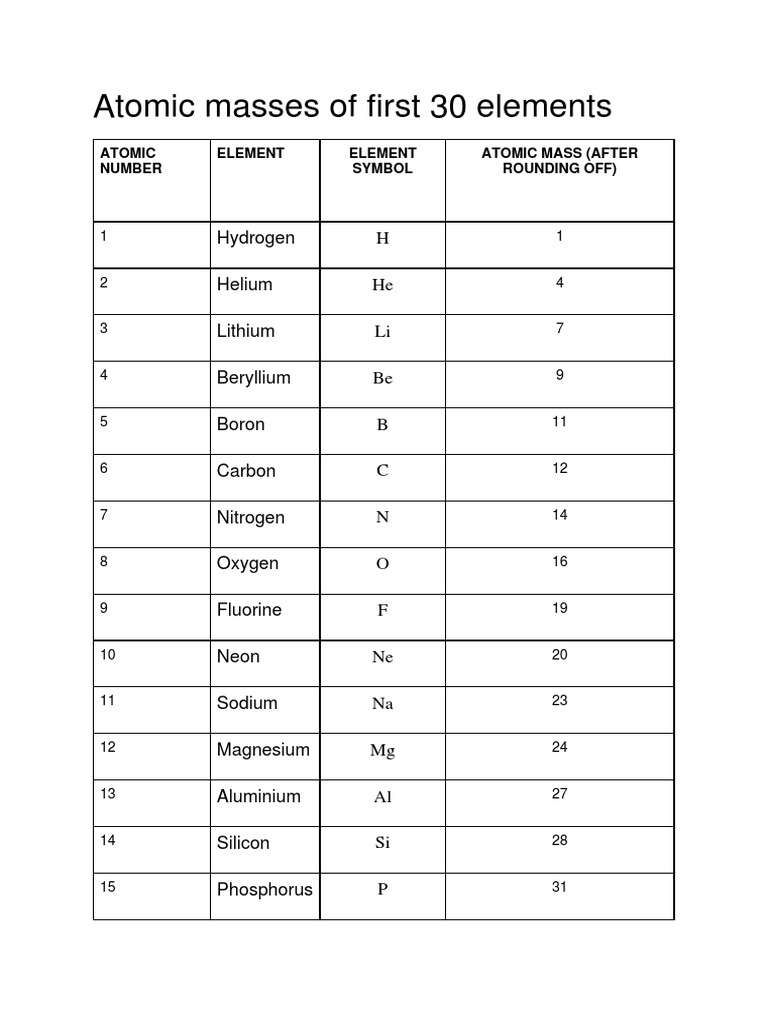
Approx atomic mass of first 30 elements
The atomic mass of elements is measured with the help of unified atomic mass units, approx atomic mass of first 30 elements. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next.
Approx atomic mass of first 30 elements
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Since we have seen the definition of atomic mass let us discuss it in detail. The atomic mass of a solitary atom is its absolute mass and is regularly expressed in atomic mass units or amu. For example, a normal carbon atom with six neutrons and six protons is denoted as carbon It has an atomic mass equal to 12 amu. The atomic mass number is usually rounded off to the nearest whole number. Since an element's isotopes have distinctive atomic masses, researchers may likewise decide the general atomic mass—once in a while called the atomic weight—for an element. The general atomic mass is the normal of the atomic masses of the apparent multitude of various isotopes in an example. Every isotope's contribution to the normal is controlled by how huge a fraction of the example it makes up. The overall atomic masses that are given in periodic tables like the one for hydrogen are determined for the naturally occurring isotopes of each element, weighted by the weight of those particular isotopes on earth. The atomic number gives a number of how many protons are inside the nucleus of the atom. Elements are identified based on the number of protons in the nucleus regardless of the number of neutrons present.
Before going into atomic mass, it is essential to learn about isotopes. Atomic mass is denoted by the letter A. It is expressed as a decimal fraction.
Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. One dalton is equivalent to one-twelfth of the mass of a carbon atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. This relationship helps simplify calculations and understanding of atomic masses.
As early chemists worked to purify ores and discovered more elements, they realized that various elements could be grouped together by their similar chemical behaviors. One such grouping includes lithium Li , sodium Na , and potassium K : These elements all are shiny, conduct heat and electricity well, and have similar chemical properties. A second grouping includes calcium Ca , strontium Sr , and barium Ba , which also are shiny, good conductors of heat and electricity, and have chemical properties in common. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and K are much more reactive than are Ca, Sr, and Ba; Li, Na, and K form compounds with oxygen in a ratio of two of their atoms to one oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen atom. Fluorine F , chlorine Cl , bromine Br , and iodine I also exhibit similar properties to each other, but these properties are drastically different from those of any of the elements above.
Approx atomic mass of first 30 elements
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg. They differ only because a 24 Mg atom has 12 neutrons in its nucleus, a 25 Mg atom has 13 neutrons, and a 26 Mg has 14 neutrons. Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2 H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3 H, is also called tritium and sometimes symbolized T. Use this Build an Atom simulator to build atoms of the first 10 elements, see which isotopes exist, check nuclear stability, and gain experience with isotope symbols. Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number a whole number. However, the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes.
Fab 500
Adenike nikky November 27, at am. The relation between the atomic mass and the number of moles is that, when measured, the amu directly gives a measure in grams of the element present in one mole. Balgopal October 16, at am. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Please go through our recently updated Improvement Guidelines before submitting any improvements. Let us take a look at what is the atomic mass of elements from 1 to Login To View Results. I liked this app and fell very easy in studies. The atomic number is simply a digit which is used for placing the elements in the periodic table. Similarly, the molecular mass of water is Save Article Save.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
Balgopal October 16, at am. What is one amu? What Is A Chemical Equation. Iupac Nomenclature Rules. Carousel Next. The mass a molecule carries is known as its molecular mass, additionally known as molecular weight. The atomic number can also be called atomic weight. Atomic mass is also used in the classification of different isotopes of the same element. Trending in News. What is the Atomic Mass of Boron? Submit your entries in Dev Scripter today. Atomic Mass.

Very useful idea