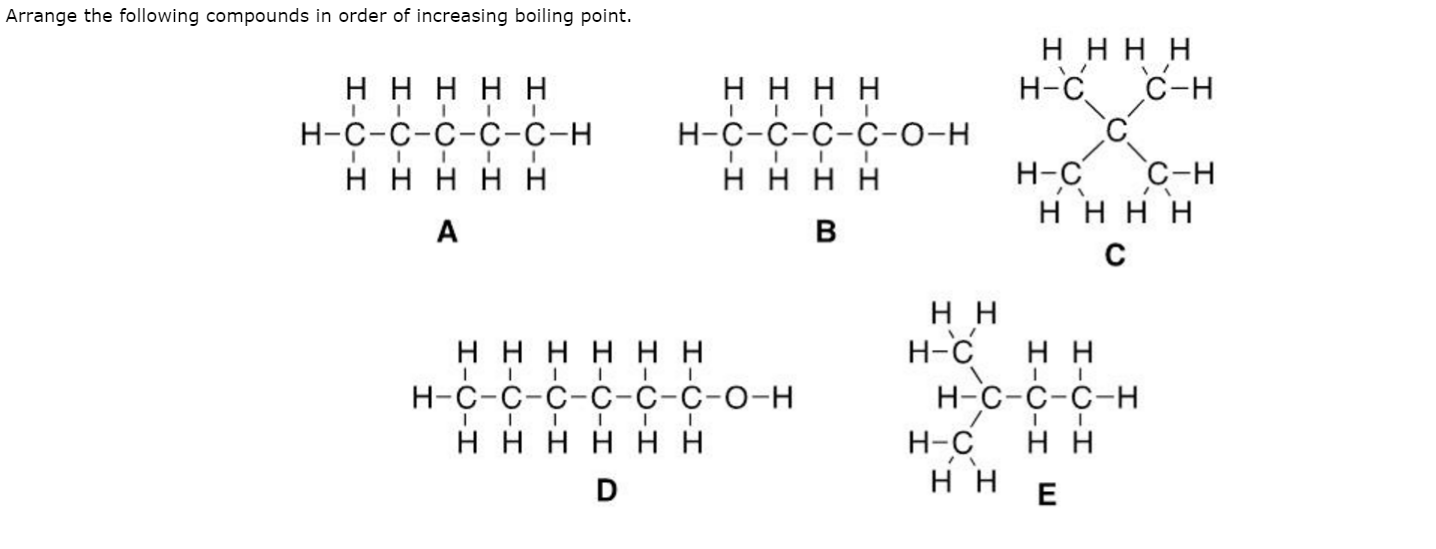
Arrange the following in the increasing order of boiling point
Arrange the following : In increasing order of basic strength : a Arrange the following : In decreasing order of basic strength in gas
Alcohol has the highest boiling point due to more extensive intermolecular H-bonding. Aldehyde is more polar than ether so, ethanal has high BP than ethyl ether. And alkane has the lowest BP. Dont't have an account? Register Now.
Arrange the following in the increasing order of boiling point
Do you know how bromomethane bromoform chloromethane and chloroform are arranged in order of increasing boiling point? Learn the concept of intermolecular forces and their effect on boiling point with Toppr, the best online learning platform for students. Ask any organic chemistry question and get expert answers. Continue reading Arrange the compounds in decreasing order of boiling point. Arrange the following compounds in increasing order of their boiling point. Arrange the following compounds in the order of properties indicated a Pentanol Pentanol Ethanol Propanol and Methanol. See increasing order of boiling point Solution Q 3 Continue reading Problem 6. The molecular mass of neon and HF is almost the same. Explain why the boiling points of neon and HF are different. Previous question Next question. Arrange the following compounds in order of their increasing number of points: A Pentanol butanol butanol ethanol propanol methanol B Pentanol n-butane pentanal ethoxyethane Continue reading Skip to content Home arrange the following compounds in increasing order of their boiling points.
Explain why the boiling points of neon and HF are different. Hospitality and Tourism Change. Do you know how to arrange bromomethane chloromethane and chloroform in order of increasing boiling point?
Do you know how to arrange bromomethane chloromethane and chloroform in order of increasing boiling point? Learn the concept of intermolecular forces and their effect on boiling point with Toppr the best online learning platform for students. Ask any Organic Chemistry question and get answers from experts. Continue reading Arrange the compounds in order of decreasing boiling point. Arrange the following compounds in order of increasing boiling point.
Arrange the following sets of compounds in order of their increasing boiling points: A : Pentanol, butanol, butanol, ethanol, propanol, methanol B : Pentanol, n-butane, pentanal, ethoxyethane. Number of carbon atoms: As the number of carbon atoms increases in alcohol molecule, the boiling point of alcohol also increases as the molecular mass increases which increases the molecular attraction between molecules. Branching in molecules: As the number of branching increases, the boiling point of alcohol decreases because branching in molecules decreases the surface area thereby decreasing the attractive forces between individual molecules. Hydrogen bonding: As the extent of hydrogen bonding increases, the boiling point also increases due to increase in the attractive forces between individual molecules. Moreover, dipole-dipole interactions are also similar in both the compounds only hydrogen bonding is making a difference as extent of intermolecular hydrogen bonding is greater in case of pentanol than pentanal. Hence, boiling point of pentanol is greater than pentanal. Arrange the following compounds in increasing order of boiling point. Propan - 1 - ol, butan - 1 - ol, butan - 2 - ol, pentan - 1 - ol a Propanol, butanol, butan - 1 - ol, pentano b Propanol, butan - 1 - ol, butanol, pentanol c Propanol, butanol, butanol, pentanol d Propanol, butanol, butanol, pentanol.
Arrange the following in the increasing order of boiling point
Arrange each of the following sets of compounds in order of increasing boiling point temperature:. Download Guided Solution as a pdf. The stronger the intermolecular forces, the more energy required to break the intermolecular forces and transition the compound between the liquid phase and the gas phase. All forces are the same type therefore it goes based on size. The largest atom has the highest boiling point temperature. All are nonpolar with dispersion forces. All molecules have the same elements but differ in the numbers of C and H and the molecule size. The larger the molecule larger size, molar mass and surface area , the higher the boiling point temperature.
Cruella deville outfit
Arrange the following compounds in increasing order of their boiling point. How will you convert : Methanol to ethanoic acid Text Solution. How will you convert : Methanamine into ethanamine. In how many years will it become 16 times itself? Aldehyde is more polar than ether so, ethanal has high BP than ethyl ether. In other words, the boiling points of alcohols are higher than those of amines of comparable molecular masses. Arrange the following sets of compounds in order of increasing boiling point A Pentanol butanol butanol ethanol propanol methanol B Pentanol n-butane pentanal ethoxyethane Continue reading How will you convert : Ethanamine into methanamine. Accomplish the following conversions : Aniline to 2, 4, 6-tribromofl Further, the extent of H-bonding depends upon the number of H-atoms on the N-atom. Ask any Organic Chemistry question and get answers from experts. How will you convert : Nitromethane into dimethylamine. Your Answer. View Solution. Arrange each compound in order of increasing boiling point
As you know, a molecule's boiling point depends on the strength of the intermolecular forces of attraction its molecules exhibit. In your case, you have to find how the boling points of three nonpolar molecules relate to each other.
Solved Arrange these compounds in increasing order Arrange the following compounds in increasing order of Electronegativity of O is higher than that of N, therefore, alcohols from stronger H-bonds than amines. Arrange the following sets of compounds in order of increasing boiling point A Pentanol butanol butanol ethanol propanol methanol B Pentanol n-butane pentanal ethoxyethane Continue reading Arrange each of the compounds in order of increasing boiling Quick links BTech M. Arrange these compounds in descending order Option: 1 1 A certain loan amounts, under compound interest, compounded annually earns an interest of Rs. By admin. Mobile No. Latest Question A sum of money under compound interest doubles itself in 4 years. Colleges Colleges Accepting B.

0 thoughts on “Arrange the following in the increasing order of boiling point”