Arsenic molar mass
Molar mass of As 4 O 6 is Then, lookup atomic arsenic molar mass for each element in periodic table : As: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
Arsenic is a chemical element ; it has symbol As and atomic number Arsenic occurs in many minerals, usually in combination with sulfur and metals , but also as a pure elemental crystal. Arsenic is a notoriously toxic metalloid. It has various allotropes , but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead for example, in car batteries and ammunition. Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III—V compound semiconductor gallium arsenide.
Arsenic molar mass
Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below. Group A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Block Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp s , principal p , diffuse d , and fundamental f. Atomic number The number of protons in an atom. Electron configuration The arrangements of electrons above the last closed shell noble gas.
This unstable allotrope, being molecular, is the most volatile, arsenic molar mass, least dense, and most toxic. Bibcode : WASP. This may find applications in areas where the potable water is extracted from underground aquifers.
Molar mass of Arsenic As is Then, lookup atomic weights for each element in periodic table : As: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Molar mass of Arsenic As is Then, lookup atomic weights for each element in periodic table : As: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in As: As: 1 Then, lookup atomic weights for each element in periodic table : As: Arsenic is a brittle, silver-gray metalloid that can also appear in various forms as a yellow, white, or black powder. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
Arsenic molar mass
Arsenic is a chemical element ; it has symbol As and atomic number Arsenic occurs in many minerals, usually in combination with sulfur and metals , but also as a pure elemental crystal. Arsenic is a notoriously toxic metalloid. It has various allotropes , but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead for example, in car batteries and ammunition. Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III—V compound semiconductor gallium arsenide.
Moto gp tv times
Archived from the original PDF on 2 May One mole contains exactly 6. Read Books. Key isotopes. The World Health Organization report that arsenic in drinking water could end up causing between , to , deaths in Bangladesh from cancer. Chinese Physics C. Redox reactions involving Fe also appear to be essential factors in the fate of arsenic in aquatic systems. It is sensitive to mobilization at pH values typical of natural waters pH 6. This is where the artist explains his interpretation of the element and the science behind the picture. Weinheim: Wiley-VCH. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. It sounds like a Doctor Who monster and in a number of ways this element does have a few properties that would make it suitable for any good, outer space sci-fi horror movie. Inorganic arsenic and its compounds, upon entering the food chain , are progressively metabolized through a process of methylation. Arsenic is used as the group 5 element in the III-V semiconductors gallium arsenide , indium arsenide , and aluminium arsenide. The name is thought to come from 'arsenikon', the Greek name for the yellow pigment orpiment.
Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Inorganic arsenic compounds are found in soils, sediments, and groundwater.
Values are given for typical oxidation number and coordination. Synthetic arsenates include Scheele's Green cupric hydrogen arsenate, acidic copper arsenate , calcium arsenate , and lead hydrogen arsenate. Read Edit View history. See how this site uses Cookies. I'm Chris Smith, thank you for listening and goodbye. Haynes, ed. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Retrieved 14 June Arsenic is bioaccumulative in many organisms, marine species in particular, but it does not appear to biomagnify significantly in food webs. Y Yttrium Ytterbium. Bibcode : WASP.. Bioremediation requires careful evaluation and design in accordance with existing conditions. Food and Drug Administration set the "level of concern" for inorganic arsenic in apple and pear juices at 23 ppb, based on non-carcinogenic effects, and began blocking importation of products in excess of this level; it also required recalls for non-conforming domestic products.

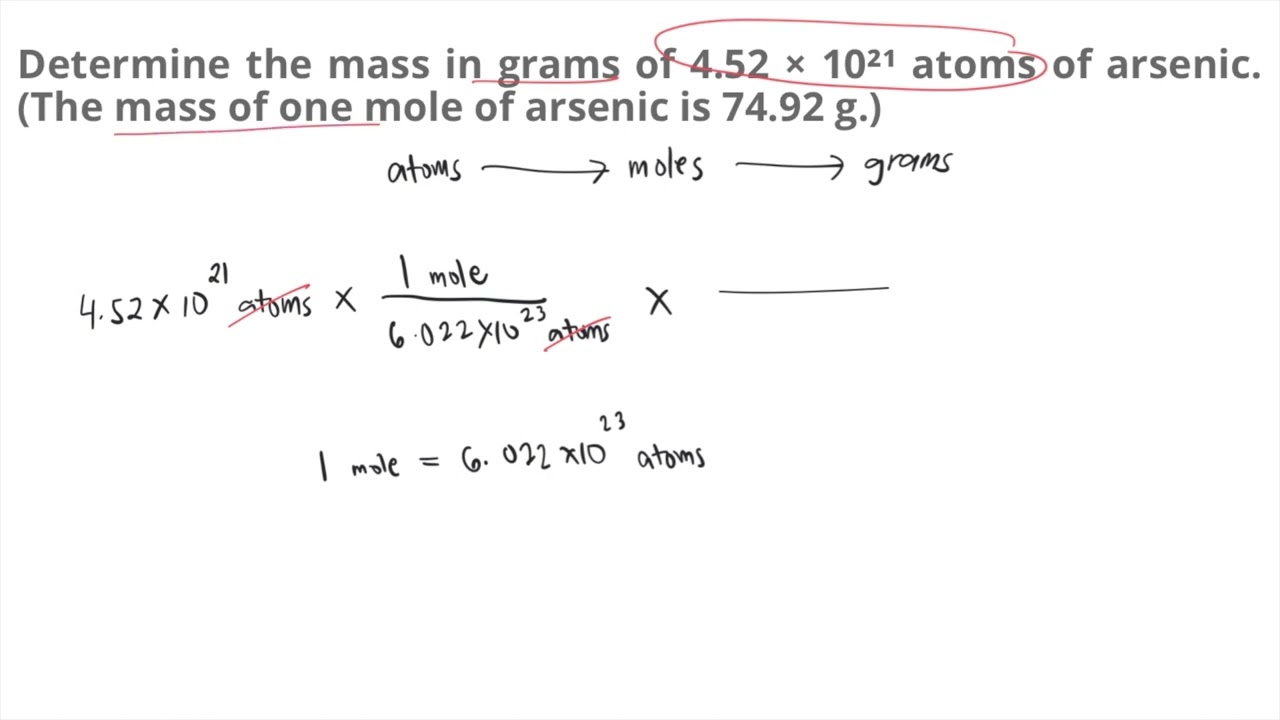
You have thought up such matchless answer?