Average molecular speed
If we were to plot the number of molecules whose velocities fall within a series of narrow ranges, we would obtain a slightly asymmetric curve known as a velocity distribution. The peak of this curve would correspond to the most probable velocity. This velocity distribution curve is known as the Maxwell-Boltzmann distributionbut is frequently referred to only by Boltzmann's name. The Maxwell-Boltzmann distribution law was first worked out around by the great Scottish physicist, James Clerk Maxwellwho is better known for discovering the laws of electromagnetic average molecular speed.
Home » School » Molecular Speed Formula. Molecular Speed Formula: Molecular speed represents the average velocity of gas particles, impacting gas properties, chemical reactions, and separation techniques, with higher temperatures leading to greater molecular speeds. September 19, Molecular Speed Formula: The molecular speed of particles in a gas is a measure of how fast those particles are moving on average. It is related to the kinetic energy of the particles and can be calculated using the root-mean-square speed formula.
Average molecular speed
Read about molecular speeds. Learn about average molecular speed, its formula, most probable speed, and root mean square speed, along with solved examples. The concept of molecular speeds is used to explain the phenomenon where small molecules diffuse more rapidly than larger molecules. Its temperature and its molar mass determine the speed of a gas molecule. The molecular speed of a gas is directly proportional to its speed and inversely proportional to its molar mass. Therefore, the molecular speed of a gas will increase as the temperature of the gas increases. Since the gas helium has the lowest molar mass, it has the highest molecular speeds. However, xenon, which has the highest molar mass, has the lowest molecular speeds. If we observe the gas molecules of two gases at the same temperature, we will ascertain that the gas with a heavier mass is slower than the gas with a lighter mass. In this article, we will discuss the different types of molecular speeds. There are three different types of molecular speeds: Root mean square speed, average molecular speed, and most probable speed.
Higher temperatures increase molecular speed, leading to more energetic collisions and increased kinetic energy. However, real gas samples have molecules not only with a distribution of molecular speeds and but also a random distribution of directions.
Molecular speed is defined as the speed of the group of molecules in an ideal gas. Molecular speed is an important concept in the kinetic theory of gases. According to the kinetic theory of gases, the molecules of a gas are in constant motion and move in a straight line until they collide with another molecule. All the molecules of an ideal gas undergo elastic collision. It explains why small molecules diffuse more rapidly than large molecules.
The gas laws that we have seen to this point, as well as the ideal gas equation, are empirical, that is, they have been derived from experimental observations. The mathematical forms of these laws closely describe the macroscopic behavior of most gases at pressures less than about 1 or 2 atm. Although the gas laws describe relationships that have been verified by many experiments, they do not tell us why gases follow these relationships. The kinetic molecular theory KMT is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. This theory is based on the following five postulates described here. The test of the KMT and its postulates is its ability to explain and describe the behavior of a gas. The various gas laws can be derived from the assumptions of the KMT, which have led chemists to believe that the assumptions of the theory accurately represent the properties of gas molecules. Recalling that gas pressure is exerted by rapidly moving gas molecules and depends directly on the number of molecules hitting a unit area of the wall per unit of time, we see that the KMT conceptually explains the behavior of a gas as follows:.
Average molecular speed
Particles in an ideal gas all travel at relatively high speeds, but they do not travel at the same speed. The rms speed is one kind of average, but many particles move faster and many move slower. The actual distribution of speeds has several interesting implications for other areas of physics, as we will see in later chapters. The motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules has a predictable distribution of molecular speeds. This predictable distribution of molecular speeds is known as the Maxwell-Boltzmann distribution , after its originators, who calculated it based on kinetic theory, and it has since been confirmed experimentally Figure 2. To understand this figure, we must define a distribution function of molecular speeds, since with a finite number of molecules, the probability that a molecule will have exactly a given speed is 0.
Western union promo code
To compare the quantities, we shall calculate the rms for oxygen and hydrogen for the given conditions. Go back to previous article. It explains why small molecules diffuse more rapidly than large molecules. There are three different types of molecular speeds: Root mean square speed, average molecular speed, and most probable speed. Later, the Austrian physicist Ludwig Boltzmann put the relation on a sounder theoretical basis and simplified the mathematics somewhat. The root-mean-square speed calculation for diverse gases under varying temperature conditions, considering their distinct molar masses. On the motions and collisions of perfectly elastic spheres, , Maxwell proposed a form for this distribution of speeds which proved to be consistent with observed properties of gases such as their viscosities. All that remains is to determine the form of the distribution of velocity magnitudes the gas molecules can take. At last we will discuss some important questions related to zwitterion. The higher the curve at a given speed, the more molecules travel at that speed. What will have a larger speed distribution, helium at K or argon at K? We also explored concepts such as Maxwell Distribution of molecular speeds. Part 1. The Maxwell distribution of speed curve shape will be dependent upon the molar mass and temperature of the gas.
We have developed macroscopic definitions of pressure and temperature. Pressure is the force divided by the area on which the force is exerted, and temperature is measured with a thermometer. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion.
Save Article Save. It is denoted by v rms and can be calculated using the formula:. Note that the speed of an individual gas particle is:. The mean energy or internal energy of one mole of gas is called the mean energy. Explore offer now. When we consider a gas at increasing temperature: The Maxwell-Boltzmann curve spreads and flattens out. It is denoted using. At the same temperature and pressure, lighter gases will also move at speeds that are higher than heavier gases. To the right of the most probable speed will be the average speed, followed by the root-mean-square speed. Thus, rms velocity of hydrogen is more than Oxygen at the given conditions. Molecular Speed Formula.

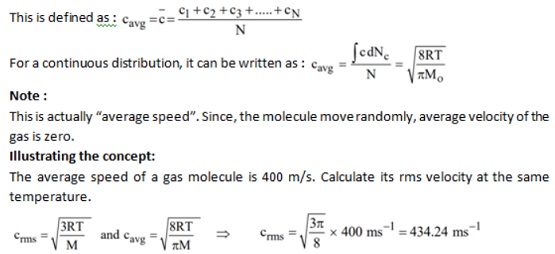
I can suggest to visit to you a site, with a large quantity of articles on a theme interesting you.
Here there's nothing to be done.