B lewis structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those b lewis structure electrons would be useful.
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron configuration, i. Lewis structure does NOT attempt to explain the geometry of molecules, how the bonds form, or how the electrons are shared between the atoms. It is the simplest and most limited theory on electronic structure.
B lewis structure
Together the three resonance structures suggest partial double-bond character in the Be-X bond, which results in an intermediate bond length between a single and double bond. There are issues with each of these resonance structures. The structure in the middle is a mix of these problems. None of these situations is ideal according to Lewis theory. In contrast to BeF 2 , solid BeCl 2 is a 1-dimensional polymer consisting of edge-shared tetrahedral. In the gas phase, BeCl 2 exists as a dimer with two chlorine atoms bridging two Be atoms. In the dimer, the Be atoms are 3-coordinate. Bridging Cl atoms are two-coordinate, while terminal Cl atoms are one-coordinate. At higher temperatures in the vapor phase, the linear monomer also exists. The structure with only single bonds is the most common representation for this molecule because the charge separation shown in the other structures is considered to be unfavorable. The highly polarized B-F bond has a dipole moment that lies opposite to the indicated formal charges shown in the resonance structures with double bonds between boron and fluorine. The case is similar to structures of other boron trihalides as well.
Group 15 elements such as nitrogen have b lewis structure valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. Chemical structures may be written in more compact forms, particularly when showing organic molecules.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. Lewis Structures We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions.
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures. This section will discuss the rules for writing out Lewis structures correctly. Writing out Lewis structures can be at times, tricky and somewhat difficult. A compound can have multiple Lewis Structures that contribute to the shape of the overall compound, so one Lewis structure of a compound may not necessarily be exactly what the compound looks like.
B lewis structure
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 7. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:.
1100 words you need to know pdf
Add lone pairs of electrons on the terminal atoms until their octet is complete or you run out of electrons. Why are there different ways for the "same" Lewis structure? Odd-electron Molecules We call molecules that contain an odd number of electrons free radicals. Here are some steps on how to draw lewis dot diagram of a moleculeto follow while drawing a lewis dot structure of any molecule:. You have determined the "best" Lewis structure octets completed and lowest formal charges for NO 3 - , but there are a number of ways to show this structure. The law states that atoms will tend to bond so that they each achieve a full outer shell of electrons an octet. Connect each atom to the central atom with a single bond one electron pair. For a molecule, we add the number of valence electrons on each atom in the molecule:. A single shared pair of electrons is called a single bond. The octet rule is a powerful predictor of molecular structure, but it is not always perfectly accurate.
A Lewis structure shows the bonding between atoms as short lines some books use pairs of dots and non-bonding valence electrons as dots. To learn about Lewis structures, we will start with the Lewis symbol.
It is also common to show only the net charge on the ion rather than all of the formal charges, i. In general, the less electronegative elements are more likely to be central atoms. With the remaining electrons, fill the octet for the central atom. If the central atom has more than eight electrons and is not one of the octet rule exceptions, the number of valence electrons in Step 1 could have been incorrectly counted. In this case, there are three possible resonance structures. When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside the brackets. Now we need to add lone pairs of electrons. Rearrange the electrons to make multiple bonds with the central atom in order to obtain octets wherever possible. Figure 1. A trick is to count up valence electrons, then count up the number of electrons needed to complete the octet rule or with hydrogen just 2 electrons , then take the difference of these two numbers. Have you ever heard of the Bohr model and wondered what it is?

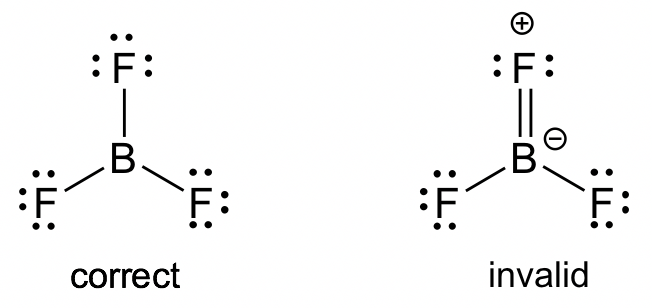
Excuse for that I interfere � At me a similar situation. Let's discuss. Write here or in PM.
In my opinion you are not right. I am assured. I can prove it. Write to me in PM.