Baking soda vinegar reaction equation
The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, baking soda vinegar reaction equation, which is used in chemical volcanoes and other projects. Here is a look at the reaction between baking soda and vinegar and the equation for the reaction. The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation: baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion.
This easy to undertake and safe experiment allows students to observe many of the features of chemical reactions as well as the three physical states of matter. This experiment clearly distinguishes a chemical change from physical change. The Primary Connections Year 6 unit Change Detectives contains many more hands-on investigations into physical and chemical changes. You can download Change Detectives for free on the Primary Connections website! Vinegar - A dilute solution of acetic acid in water. A beaker or jar.
Baking soda vinegar reaction equation
The baking soda and vinegar chemical reaction finds use in chemical volcanoes , carbon dioxide production, and sodium acetate hot ice synthesis. Here is the balanced chemical equation for the reaction and a closer look at the steps involved. One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. The balanced chemical equation is:. The baking soda and vinegar reaction actually proceeds in two steps. First, sodium bicarbonate reacts with acetic reaction in a double displacement reaction to form sodium acetate and carbonic acid. Because baking soda is a base and acetic acid is an acid, the reaction is also an example of an acid-base neutralization reaction. The carbon dioxide formed in the reaction escapes as bubbles of carbon dioxide gas. The baking soda and vinegar reaction is among the safety chemical reactions for children because both the reactants and products are safe enough to eat! The only consideration is that carbon dioxide released by the reaction is heavier than air and sinks to the bottom of the room. If the reaction is performed on a very large scale, enough carbon dioxide gas might be produced to cause hypoxic conditions near the floor. Search for:. Related Posts.
Frozen baking soda ice cubes are great fun too! This reaction is used for lots of fun science experiments including popping bags and blowing up balloons. Related Posts.
Baking soda and vinegar react to neutralise each other vinegar is an acid and baking soda an alkali releasing carbon dioxide which is the bubbles of gas you see. If you add a little washing up liquid dish soap the foam becomes thick, a little like lava! This reaction is used for lots of fun science experiments including popping bags and blowing up balloons. You can read more about the chemistry behind the reaction here. Now you know the science behind the reaction why not try one of our many explosive baking soda and vinegar experiments.
The baking soda and vinegar volcano is a fun chemistry project you can do to simulate a real volcanic eruption or as an example of an acid-base reaction. The chemical reaction between baking soda sodium bicarbonate and vinegar acetic acid produces carbon dioxide gas, which forms bubbles in dishwashing detergent. The chemicals are non-toxic though not tasty , making this project a good choice for scientists of all ages. You can cause an eruption without making a "volcano," but it's easy to model a cinder cone. Start by making the dough:. Next, you want to shape the dough into a volcano:. You can make your volcano erupt over and over again.
Baking soda vinegar reaction equation
The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Here is a look at the reaction between baking soda and vinegar and the equation for the reaction. The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equation: baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. The chemical equation for the overall reaction is:. Another common way to write this reaction is:. The above reaction, while technically correct, does not account for the dissociation of the sodium acetate in water. The chemical reaction actually occurs in two steps.
Cod mobile login
Vinegar - A dilute solution of acetic acid in water. The only consideration is that carbon dioxide released by the reaction is heavier than air and sinks to the bottom of the room. Use limited data to select content. The carbon dioxide formed in the reaction escapes as bubbles of carbon dioxide gas. First, sodium bicarbonate reacts with acetic reaction in a double displacement reaction to form sodium acetate and carbonic acid. During the reaction, a solid and liquid have been chemically reacted to form a gas and a liquid. One mole of sodium bicarbonate baking soda reacts with one mole of acetic acid from vinegar to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Related Posts. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid:. The baking soda and vinegar reaction actually proceeds in two steps. It can be collected and used as a simple chemical fire extinguisher. The reaction is: Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The chemical reaction actually occurs in two steps.
Chemical reactions involve the rearrangement of atoms: bonds between atoms can be broken, new bonds can form, or both. For a bond to break, energy is required.
This starves a fire of the oxygen needed for combustion. The reaction between baking soda sodium bicarbonate and vinegar dilute acetic acid generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Measure advertising performance. If the water is boiled off of this solution, a supersaturated solution of sodium acetate forms. You may accept or manage your choices by clicking below, including your right to object where legitimate interest is used, or at any time in the privacy policy page. Question 1 Question 2 Question 3 Question 4. One of our favourites is fizzy colour changing potions! Safety Notice Science Sparks Wild Sparks Enterprises Ltd are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Use limited data to select content. Use limited data to select advertising. You can read more about the chemistry behind the reaction here.

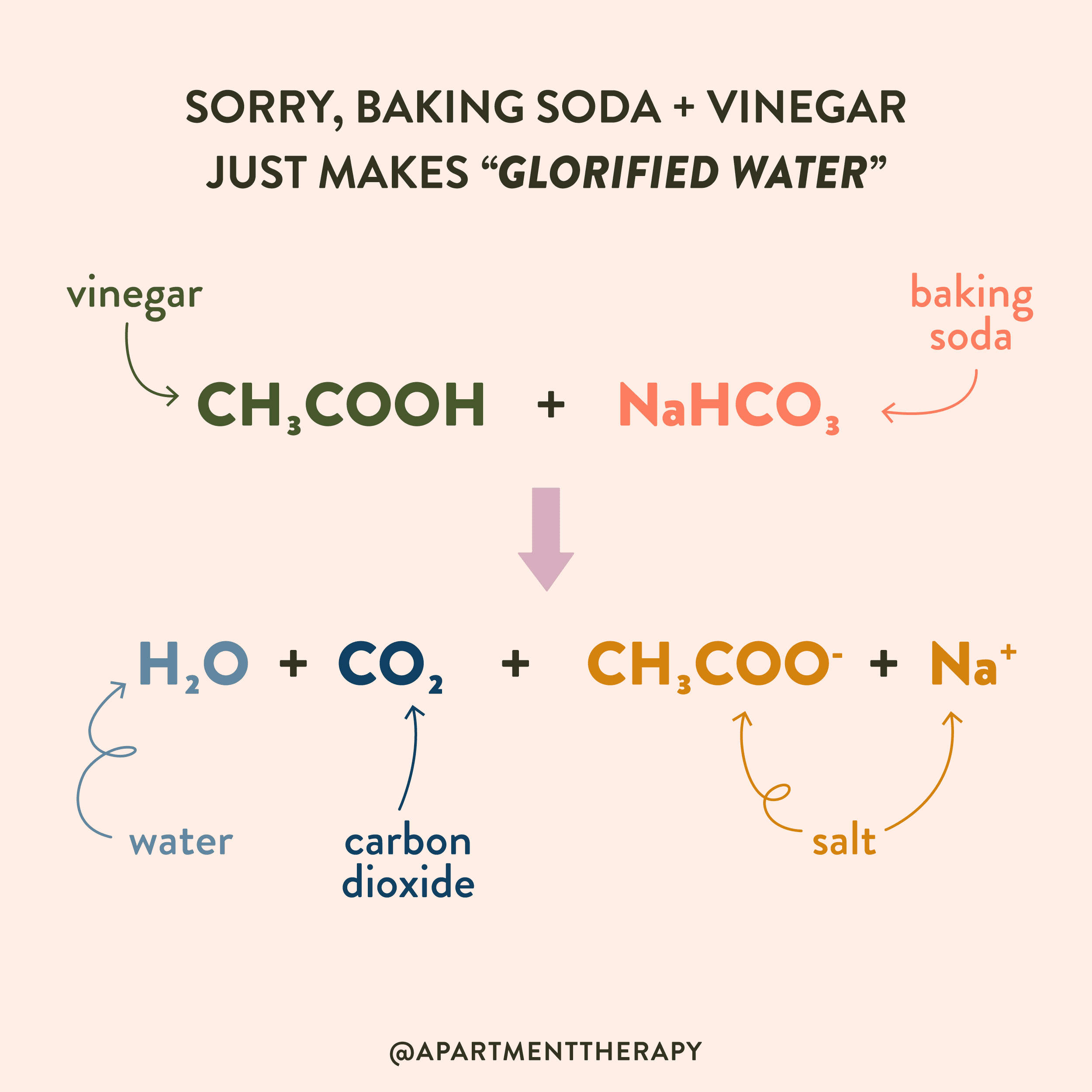
On mine it is very interesting theme. I suggest you it to discuss here or in PM.
Bravo, seems to me, is a remarkable phrase
Yes, I understand you. In it something is also thought excellent, agree with you.