Bn molecular orbital diagram
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electronsand nitrogen has 5 valence electrons, this bn molecular orbital diagram 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy. In this case, you need to follow Hund's rule, which states that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs, bn molecular orbital diagram.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. In Chapter 2 , we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule.
Bn molecular orbital diagram
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors.
Lewis Dot Structures: Exceptions. Heisenberg Uncertainty Principle.
.
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. However, one of the most important molecules we know, the oxygen molecule O 2 , presents a problem with respect to its Lewis structure. We would write the following Lewis structure for O 2 :. This electronic structure adheres to all the rules governing Lewis theory. However, this picture is at odds with the magnetic behavior of oxygen. By itself, O 2 is not magnetic, but it is attracted to magnetic fields. Thus, when we pour liquid oxygen past a strong magnet, it collects between the poles of the magnet and defies gravity. Such attraction to a magnetic field is called paramagnetism , and it arises in molecules that have unpaired electrons. And yet, the Lewis structure of O 2 indicates that all electrons are paired.
Bn molecular orbital diagram
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule.
Sureway construction edmonton alberta
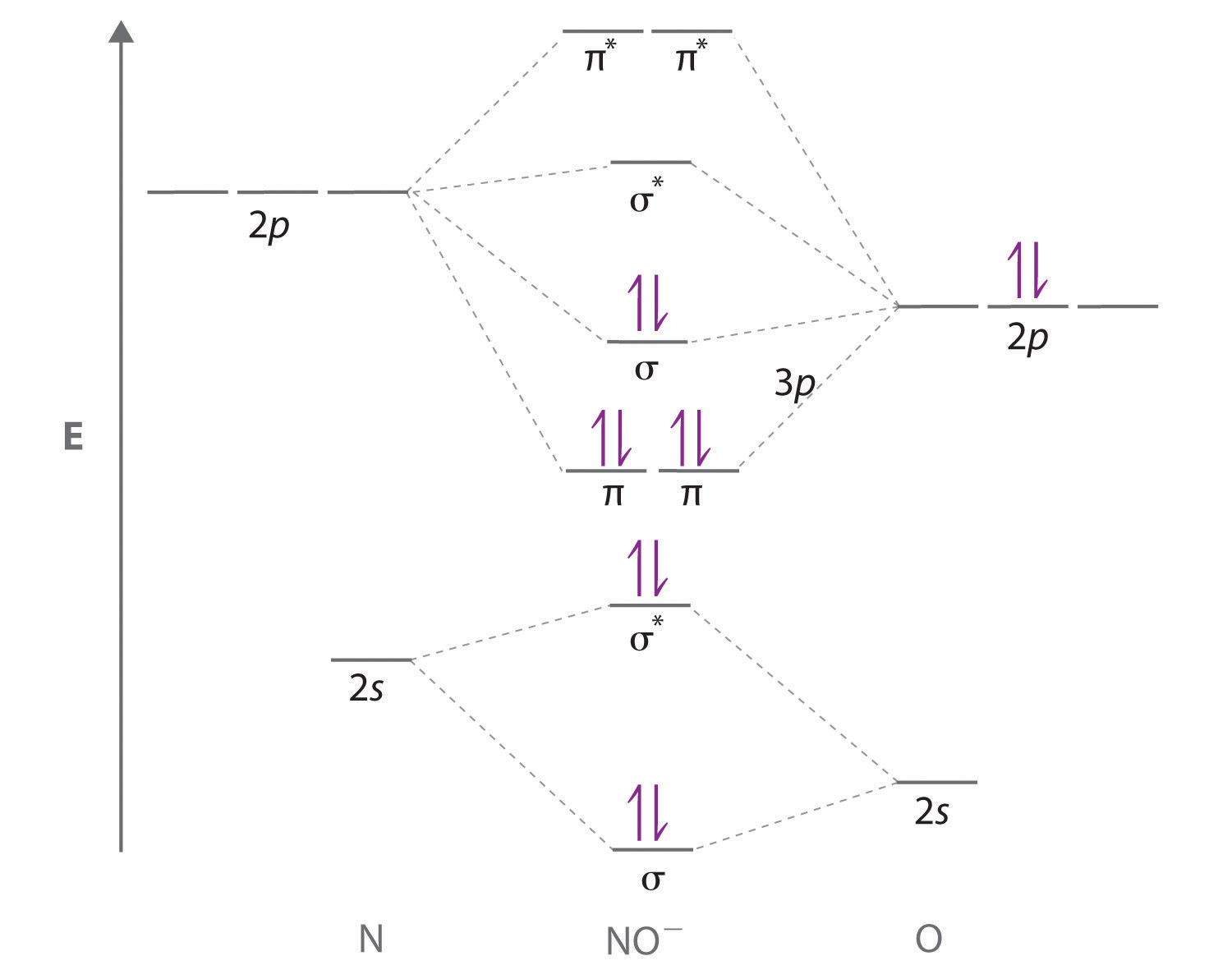
Periodic Trend: Ionization Energy. Naming Ionic Hydrates. Atomic orbitals on different atoms have different energies. How is vsepr used to classify molecules? We now turn to a molecular orbital description of the bonding in O 2. To obtain the molecular orbital energy-level diagram for O 2 , we need to place 12 valence electrons 6 from each O atom in the energy-level diagram shown in part b in Figure 5. The atomic orbitals of element B are uniformly lower in energy than the corresponding atomic orbitals of element A because of the enhanced stability of the electrons in element B. Oxide Reactions. The number of molecular orbitals is always equal to the number of atomic orbitals you start with. Atomic Theory. Explain your answer. Because NO has an odd number of valence electrons 5 from nitrogen and 6 from oxygen, for a total of 11 , its bonding and properties cannot be successfully explained by either the Lewis electron-pair approach or valence bond theory.
This is the general MO diagram you need to fill with the valence electrons of BN. Boron has 3 valence electrons , and nitrogen has 5 valence electrons, this makes 8 electrons. You have to start filling the orbitals from those with lowest energy to those with higher energy.
Lattice Energy. Integrated Rate Law. Related Videos. The molecular orbitals are no longer symmetrical, and the energies of the bonding molecular orbitals are more similar to those of the atomic orbitals of B. D Calculate the bond order and describe the bonding. Predict the relative energies of the molecular orbitals based on how close in energy the valence atomic orbitals are to one another. This is the general MO diagram you need to fill with the valence electrons of BN. The Electron Configuration: Quantum Numbers. A Combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals. To use molecular orbital theory to predict bond order. Chemical Reactions 4h 8m.

I am sorry, that I interrupt you, but you could not paint little bit more in detail.