Bohr diagram for lithium
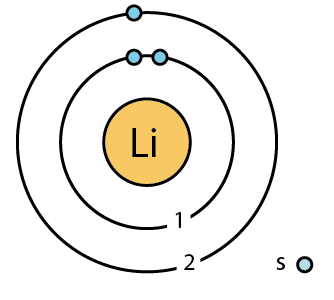
Lithium is the group 1 chemical element. It has atomic number 3 and is denoted by the symbol Li. It is a highly reactive and flammable substance due to which it is usually stored under inert conditions, mostly immersed in an inert liquid, bohr diagram for lithium, like kerosene.
From Wikimedia Commons, the free media repository. File information. Structured data. Captions Captions English Add a one-line explanation of what this file represents. I, the copyright holder of this work, hereby publish it under the following licenses:.
Bohr diagram for lithium
Top page correct Bohr model including the two-electron atoms. Lamb shift is an illusion! Our new Bohr model has suceeded in calculating the Helium ionization energy more correctly than the quantum mechanical variational methods as shown in the Top page. Lithium belongs to the alkali metal group of chemical elements, and has the atomic number 3. It is the lightest metal, and highly reactive and flammable though more stable than the other alkali metals. Naturally occurring lithium is composed of two stable isotopes, Li6 and Li7, the latter being the more abundant The ionization energies of the lithium is 5. First, suppose we have one model Fig. This value is lower than the experimental value The error is 7. But as I said in the top page, if the two electrons can be in one small orbit of one de Broglie's wavelength, this means that the ground state electron of the Bohr hydrogen-like model can come closer to the nucleus than the original orbit. And in the orbit of Fig.
They are not stagnant like other atomic particles and circulate around the nucleus, bohr diagram for lithium. In most cases, electrons fill the lower-energy orbitals first, followed by the next higher energy orbital until it is full, and so on until all electrons have been placed. So here we suppose another model as shown in Fig.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr —
Lithium is the group 1 chemical element. It has atomic number 3 and is denoted by the symbol Li. It is a highly reactive and flammable substance due to which it is usually stored under inert conditions, mostly immersed in an inert liquid, like kerosene. It is an alkali metal that exhibits metallic luster and is prone to corrosion when exposed to moisture. It usually occurs as an ionic compound such as pegmatitic minerals. Lithium can also be isolated electrolytically from potassium chloride and lithium chloride mixture. It is used in the preparation of lithium-ion batteries, making of glass, pyrotechnics, etc. This model was actually the upgraded version of the earlier model proposed by Ernst Rutherford in
Bohr diagram for lithium
Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. We can assume that if the atom is neutrally charged it would also contain 11e-. The energy shells filled up from the lowest energy to highest until all 11e- were accounted for. Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom.
Maxpreps softball
Naturally occurring lithium is composed of two stable isotopes, Li6 and Li7, the latter being the more abundant Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Similarly, neon has a complete outer 2n shell containing eight electrons. K shell can have 2, L can have 8 , M can have 18 electrons and so on. The experimental value of the ground state energy of the lithium Li atom is Atoms tend to be most stable with a full outer shell one which, after the first, contains 8 electrons , leading to what is commonly called the "octet rule". Lewis Symbols Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. The innermost shell is assigned the lowest value which increases outwards i. Bohr Model of Lithium. The electron carrying capacity of the shell also increases outwards. Save my name, email, and website in this browser for the next time I comment. The shells are also given names in numerological as well as alphabetical order. Bohr diagrams indicate how many electrons fill each principal shell. Each element, when electrically neutral, has a number of electrons equal to its atomic number.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has.
Main page Welcome Community portal Village pump Help center. This means that it is almost correct to say that the three electrons of the lithium atom are moving like Fig. It is the lightest metal, and highly reactive and flammable though more stable than the other alkali metals. We suppose de Broglie's waves are related to some limited spaces. The properties of an element are determined by its outermost electrons, or those in the highest energy orbital. Summary Description 3 Lithium Li Bohr model. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. This results shows that when the ground state energy of Li atom is Bohr Diagrams Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. English: lithium Li 3 Bohr model. So the calculation error is only 0.

I am final, I am sorry, but this answer does not approach me. Who else, what can prompt?
I think, that you are not right. I can prove it.