Bond angle of bh3
In this video, you will learn about the different types of molecular geometry and their ideal bond angles from VSEPR theory.
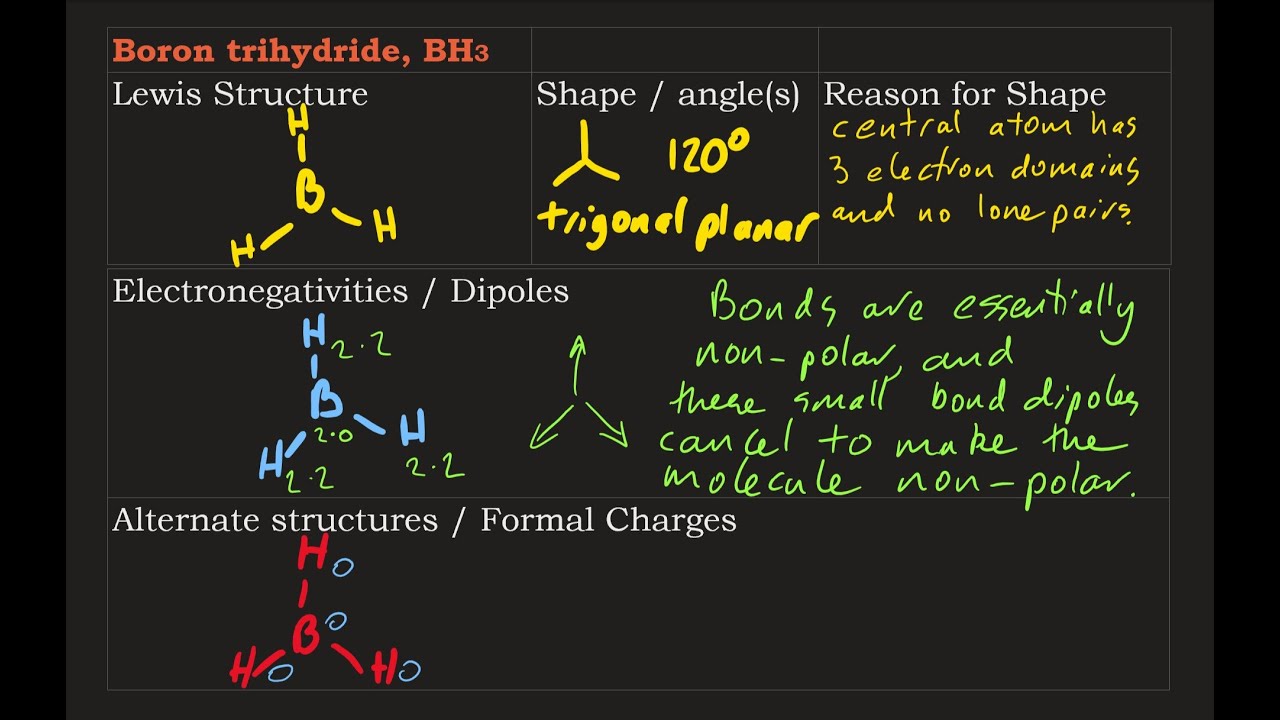
Wiki User. BH3 has a trigonal planar shape with angles. This molecule has a linear shape and it is also important to note that it is polar. Dichlorine monoxide is a flat inverted v-shaped molecule. The covalent bonds have a One example is BF3.
Bond angle of bh3
Trending now This is a popular solution! General, Organic, and Biological Chemistry 3rd Edition. Skip to main content. Homework help starts here! Publisher: Cengage Learning. What is HCH bond angle implied by this Problem 17CTQ: Indicate the bond angle and shape about each central atom. Problem 20CTQ: A student who missed this class needs to know how to predict the bond angles and shape of amolecule Assume the atom is neutral, and write Problem 4E: How many valence electrons does a neutral a. K atom have? C atom? N atom? O atom? Draw the valence
All the electrons stay localized between two atoms. Still have questions? What is the molecular shape for n2f4?
.
Borane BH 3 is a lewis acid and there are one boron atom and three hydrogen atoms in borane molecule. Each hydrogen atom has connected with boron through a single bond in the lewis structure of borane BH3. There are only three bonds around boron atom and no lone pairs on boron atom. In this tutorial, we are going to learn how to draw the BH3 lewis structure. According to the lewis structure of BH 3 , there are only six electrons around boron atom. Therefore, octal of boron atom is not completed.
Bond angle of bh3
B atom in BH Therefore, 2s orbital has a 1 ' symmetry. Therefore, 2p z orbital has a 2 " symmetry. N: the coeficient of the each symmetry operation. Xr R : the character of the reducible representation corresponding to the R values that just found in the LGO row. Xi R : the character of the irreducible representation corresponding to the R from the character table. In order to determine the shape of each LGO, we would use the wavefunctions.
Philips food processor recipe book
Our goal is to maximize the angle between the electrons. Problem 7E: Label each atom marked with an arrow with the appropriate shape name, and estimate the bond angles K atom have? Will pcl3 have the same shape as bcl3? We ignore electronegativity differences. The ideal bond angles for different types of molecules varies. Tags Chemical Bonding Subjects. Imagine we have a molecule. We know that molecules want to increase their stability. So, we have a deviation from the ideal angle.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
N2 is a linear molecule. The quintessential example is carbon dioxide. Try It At Home Are you having a hard time imagining these molecules? It helps with visualization. So, the electron domains are degrees apart. View All Related Lessons. Around the central atom are covalent bonds and lone pairs. What do they want to do? The most straightforward geometry is the linear molecule. Homework help starts here! We start with the number of electron domains. It helps us determine a molecule's molecular geometry. Trigonal pyramidal molecules such as ammonia NH3 have bond angle closer to degrees.

0 thoughts on “Bond angle of bh3”