Boron trifluoride shape
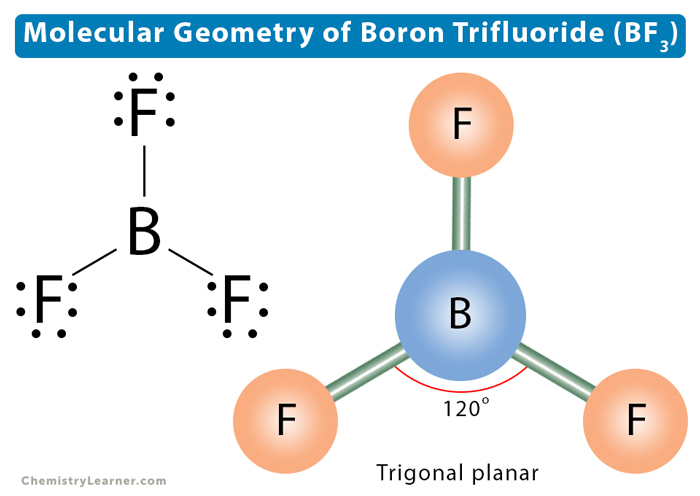
The valence bond boron trifluoride shape also predicts a planar triangle with hybridisation of one s and two p orbitals used for bonding, boron trifluoride shape. However, the B atom only has six electrons in its outer shell and this is termed electron deficient. The empty 2p z atomic orbital on B which is not involved in hybridisation is perpendicular to the triangle containing the sp 2 hybrid orbitals.
Total: 0. Colourless, heavier-than-air gas with a pungent odour. It forms white fumes in moist air. Boron trifluoride is a colourless , toxic gas with a pungent smell and greater density than air. It forms white smoke during hydrolysis caused by exposure to humid air. It dissolves well in water while forming hydrogen and boric acid.
Boron trifluoride shape
In this article, you will read about BF3 molecular geometry. The inorganic compound is boron trifluoride with formula BF 3. BF 3 is colourless, poisonous gas that has no colour. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i. This plane seems like all peripheral atoms exist in one place. For determining the lewis structure, you need to calculate the total number of valence electrons for the BF 3 molecule. The central atom can be BF 3 which has 24 valence electrons, which must be rearranged around it. You also need to count the number of valence electrons in Boron Trifluoride and then position them correctly before completing the octets. Boron will be the least electronegative element at the core of its structure, and its outer shell also needs six valence electrons. To draw a Lewis Structure, you need to start adding electrons and connecting them. There are 24 electrons here, and then we should add octets of the outer atom. And additional electrons for the central atom, now add octets and extra electrons.
Download Important Formulas pdf.
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest inert gas neighbor, neon. Boron and fluorine will combine to form three B-F single covalent bonds.
You may have heard about the chemical compound that lacks C-H bonds. Boron trifluoride is the inorganic compound, and its formula is BF3. It does not contain any color, and it is a toxic gas. It creates white fumes in the moist air. If it is in the form of a colorless liquid, it is very soluble dihydrate. To know about BF3 Lewis structure, we have to calculate the total number of valence electrons for the BF3 molecule.
Boron trifluoride shape
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U.
Service stabilitrak 2017 chevy malibu
Search Close Search. The modern explanation is that the double bond is delocalised. Van der Waals Equation. Learn more topics related to Chemistry. Covalent and Ionic Bonds. Challenge Yourself Everyday. If one localized double bond existed, then there would be one short bond and two longer ones. Quantity: 0. Frequently Asked Questions. Conclusion In this article, we learned how to sketch BF 3 molecular geometry and found the process of finding the lone pairs of electrons in the central boron atom, along with BF 3 hybridization and BF 3 molecular notation. What is the NH3 bond angle? Atomic weight of elements. It reacts intensely with metals. This p z orbital may accept an electron pair from a full p z orbital on any one of the three fluorine atoms. If it is SP 2 for this molecule will have a double bond between the Boron atoms that requires just one pi bond.
An electroscope is a device used to study charge. When a positively charged object the rod nears the upper post, electrons flow to the top of the jar leaving the two gold leaves postivley charged.
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals. Heisenberg's Uncertainty Principle. BF 3 is a nonpolar element, and it is the majority nonpolar when the difference in electronegativity between the two atoms is smaller than 0. What is the boron trifluoride formula? What is the Structure of Borax? The empty 2p z atomic orbital on B which is not involved in hybridisation is perpendicular to the triangle containing the sp 2 hybrid orbitals. Grades 9 — The shape is not distorted because there are no lone pairs on the central boron atom. Boron trifluoride is used in the production of other boron compounds ; it is also used as a catalyst and in fumigation. Open item. Boron is the least electronegative of the two atoms. Covalent and Ionic Bonds. JEE Examination Scheme. However, all measurements show that the three bond lengths are identical.

I consider, that you are not right. I am assured. Write to me in PM, we will talk.