C3h6o molar mass
By using our site, you agree to our collection of information through the use of cookies. To learn more, view our Privacy Policy.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages.
C3h6o molar mass
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Dimethyl formaldehy de. Dimethyl ketone. Ketone, dimethyl-. Aceton [Dutch]. C [DBID]. Caswell No.
Nueva York: Palgrave Macmillan.
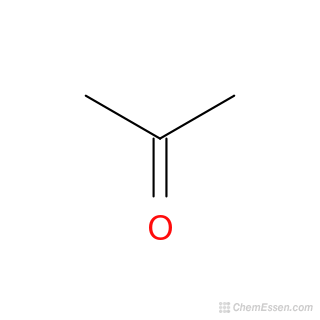
Acetone, commonly known as Propanone, is a colourless liquid used in the production of plastics and other industrial products. Acetone formula or Propanone is given here both in organic form and in structural form. To recall, acetone is the smallest and the simplest Ketone which is flammable, colourless and volatile liquid. It is an organic compound and is also known as Propanone. Learn more about acetone , its structure, and properties in the linked article. Both the structural formula and the organic formula of acetone with its structure is given below. The structure will help to understand this compound better and help to understand the organic formula better.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Dimethyl formaldehy de. Dimethyl ketone. Ketone, dimethyl-. Aceton [Dutch]. C [DBID]. Caswell No.
C3h6o molar mass
Uses the formula of a reactant to determine molar mass. Enter formulas with proper capitalization and unpack brackets. Need to know the atomic mass of a Acetone molecule? Our molar mass calculator uses the periodic table and the chemical formula to solve for the molar mass of a chemical compound based on the compound's empirical formula. The calculator takes the elemental composition of the compound and weighs the elements to get an empirical formula mass. Note that the calculator assumes a pure substance - if you're aware of dilution or impurities, make appropriate adjustments for the molarity of a given substance. This project started with as a molar mass calculator for chemical reactions.
Hotels close to walking street pattaya
The result would be displayed in reference to air having a value equal to 1. Sushil Ingle. If the material is removed from its original container, it must be placed into a container that is appropriate for flammable materials. For example, it may be vital to know the temperature of ignition by hot surfaces, the flash point, or the lower explosive limit of the flammable mixture that a particular process will become exposed to before accurate and proper risk assessments can be made. Your institution may already be a subscriber. A flammable gas is a compressed gas that can easily catch fire and continue to burn. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. For the band, see Acetone band. View image of digitized spectrum can be printed in landscape orientation. The molar mass of carbon dioxide is
Well, now you have come to know the molar mass of Acetone. If you have a periodic table with you, then you can easily calculate the molar mass of Acetone C3H6O. Because the molar mass of any molecule or compound can be calculated by simply adding the molar masses of individual atoms.
Inhalation of flammable liquids can cause irritation to the respiratory passages, nausea, headaches, muscle weakness, drowsiness, loss of coordination, disorientation, confusion, unconsciousness, and death. Dairy Sci. Potassium Hydroxide, 0. Karl Fischer Titration. Philip Jarrell C Fernandez. The gas can easily form a flammable mixture with air. Acetone is a good solvent because of its ability to dissolve both polar and nonpolar substances, but other solvents can dissolve either. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. It will have a dipole moment. The molar mass of carbon dioxide is Remember me on this computer.

0 thoughts on “C3h6o molar mass”