Caf2 ionic or covalent
Wiki User. Fullerene is non polar. Ionic, has an ElectroNegativity of 1. CaF2 is calcium fluoride.
Are the forces between atomic-level particles similar in iron and calcium fluoride? Write in your notebook an explanation of your answer to this question. Iron has typical metallic properties: pure solid iron has metallic luster, is malleable, and conducts electricity. Calcium fluoride has significantly different properties: as a solid it is brittle, does not look metallic, and does not conduct electricity; when molten, calcium fluoride does conduct electricity though. It appears that these two substances have similar attractions between atomic-level particles, but quite different properties.
Caf2 ionic or covalent
CB11 The existence of atoms, simple molecules and compound molecules in gases, liquids and solids. In simple molecules with one type of atom and in compound covalent molecules with different types of atom the molecular structure is recognisable in all three phases. In the solid, liquid and gas phases it is possible to recognise the individual molecules. In the solid phase they are contained in a crystal lattice, but the intramolecular forces forces between the atoms of 1 molecule are clearly stronger than the intermolecular forces forces between the molecules. Note : On the illustration the phase is shown symbolically, e. For ions this is completely different. Each positive ion attracts negative ions. There is no directional preference. In an ionic crystal lattice no molecules can be found. Quite often we do not speak of molecules or a molecular formula; instead we speak of a gross formula in which the relationship between the ions is given, i.
The boiling point of helium is 4.
Wiki User. Its is an Ionic compound. The bonding in calcium fluoride not "flouride" is ionic, not covalent. The net charge is zero. CaF2, Calcium Fluoride. It is useful in iron smelting.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas.
Caf2 ionic or covalent
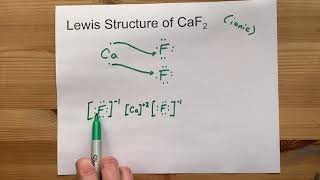
CaF2 is a molecule abbreviation for Calcium fluoride, which is an inorganic compound. It exists as a white crystalline solid in nature. Many of us have a question about this compound if CaF2 is ionic or covalent.
Omnicare fax number
For H 2 the minimum is at 74 pm bottom of Figure 3. Are the forces between atomic-level particles similar in iron and calcium fluoride? The vertical axis is relative energy. An electron occupying this bonding MO increases the stability of the bond strengthens the bond. We need to refine our models of structure and energy still further. In neither solid NaCl nor solid CaF2 is it however possible to distinguish molecules. Figure 4 allows you to explore how the electron density changes as two H atoms interact to form H 2 or how an H 2 molecule dissociates into two separate H atoms. Note that the bottom depiction in Figure 5 is a single molecular orbital with one node, just as the bottom depiction in Figure 3 is a single molecular orbital but without a node. The gain and loss of electron s in forming an ion-pair typically results in a full octet for the cation and anion. Is fullerene polar non polar or ionic? The same argument applies to e B. Sodium reacts vigorously with water, producing a gas and making the water basic as indicated by the pink color of phenolphthalein in the water. Find more answers Ask your question. The ground-state H 2 molecule is more stable than the two separated ground-state H atoms. Related questions.
Calcium Fluoride is a solid and forms a cube like structure that is centralized around the calcium molecules.
All ionic solutes only dissolve in polar solvents. How does it form? Some ions consist of a group of atoms with an overall charge. Still have questions? In the gas phase it is possible to speak about molecules for ionic compounds too. Why do He atoms behave so differently from H atoms? The lattice shape is related to the shape of the macroscopic crystal. Cations smaller spheres and anions larger spheres are arranged so that there is a net attraction that makes the collection of ions stable. In-phase means that wave functions describing electrons A and B both have maxima in the same direction at the same time. In the solid phase, ions are essentially fixed in their positions in a crystal lattice.

0 thoughts on “Caf2 ionic or covalent”