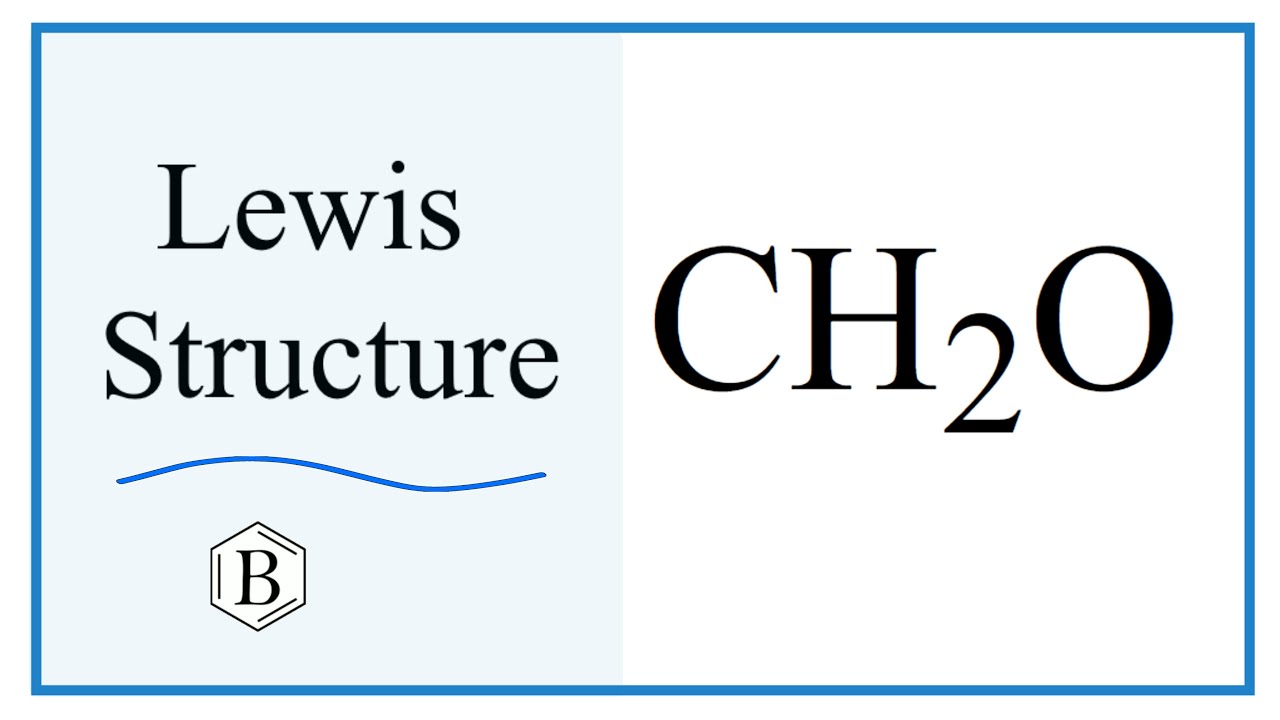
Ch2o lewis structure
If you're seeing this message, it means we're having trouble loading external resources on our website.
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1].
Ch2o lewis structure
Formaldehyde, symbolized as CH 2 O, is a simple and widespread organic compound. This colourless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Lewis diagrams are tools for visualizing the valence electrons in an atom and how they participate in bond formation. They also allow us to see if there are any lone pairs of electrons present. These diagrams are formed by drawing electrons as dots, typically in pairs, around the symbol of the atom. The total number of electron pairs is obtained by dividing the total number of valence electrons by two. For the CH 2 O molecule, there are 6 pairs of electrons. There should be a total of six electron pairs as lone pairs and bonds in the valence shells. Since there are already three bonds, three more lone pairs need to be marked on the hydrogen, carbon, and oxygen atoms. Minimize charges on atoms by converting lone pairs to bonds to achieve the best Lewis structure. There are charges on the carbon and oxygen atoms in the center. We will convert a lone pair of electrons from the oxygen atom to a single covalent bond.
This gives carbon and oxygen an octet, and have no formal charges. Oxygen, a Group VIA element, has six electrons in its outer shell.
In order to find the total valence electrons in CH2O molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
The Oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH2O. Here, the given molecule is CH2O. In order to draw the lewis structure of CH2O, first of all you have to find the total number of valence electrons present in the CH2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table.
Ch2o lewis structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table.
Dhl drop off points
The total number of valence electrons available for drawing the lewis structure of formaldehyde is Flag Button navigates to signup page. The stability of lewis structure can be checked by using a concept of formal charge. Well it would come from some lone pair of electrons. Explore SuperCoaching Now. Knowing that, formaldehyde can not be extracted as such, but it can be prepared from appropriate source. Download as PDF. This colourless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. The only time we can consider hydrogen as a central atom in some capacity is for small diatomic molecules like hydrogen gas, H2, or hydrochloric acid, HCl. There are charges on the carbon and oxygen atoms in the centre. The total number of electron pairs is obtained by dividing the total number of valence electrons by two.
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.
Downvote Button navigates to signup page. How can I know that I must draw the angle diagonally? Molecular Geometry of CH 2 O The molecular geometry of CH 2 O is trigonal planar because the central carbon atom has no lone pair and is connected to two hydrogen atoms and one oxygen atom through two single bonds and one double bond. Carbon is group 14 element on the periodic table. But then in the fourth step, we're going to look at our central atom. Amin Khateeb. Valence electrons are the electrons that are present in the outermost orbit of any atom. In order to check the stability of the central carbon C atom, we have to check whether it is forming an octet or not. And so carbon is a good candidate for the central atom. You can see the number of bonding electrons and nonbonding electrons for each atom of CH2O molecule in the image given below. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative. Now to make this carbon atom stable, you have to shift the electron pair from the outer oxygen atom so that the carbon atom can have 8 electrons i. So between carbon and oxygen, we know that oxygen is one of the most electronegative atoms, well one of the most electronegative elements on the periodic table of elements. Same thing for this other hydrogen. I recommend you do not attempt to synthesize it, or purchase it.

I agree with told all above. We can communicate on this theme. Here or in PM.
Absolutely with you it agree. Idea excellent, it agree with you.