Charge of io3
Iodate ion contains one iodine and three oxygen atoms. There is -1 charge on oxygen atom in IO 3 - lewis structure. Oxygen atoms have made bonds with center iodine atom.
Ready to learn how to draw the lewis structure of IO3- ion? Here, I have explained 6 simple steps to draw the lewis dot structure of IO3- ion along with images. The Iodine atom I is at the center and it is surrounded by 3 Oxygen atoms O. The Iodine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
Charge of io3
The Charge of IO3 Iodate ion is But the question is how can you find the charge on IO3 iodate ion? So you can easily say that the charge of IO 3 should be 1-, then only it will get canceled out. So here also you can easily say that the charge of IO 3 should be 1-, then only it will get canceled out. As seen from the above examples, The charge of IO3 is Now using the above lewis structure of IO3, you have to find the formal charge on each atom that is present in the IO3 molecule. This indicates that the IO3 Iodate ion has 1- charge. I hope you have understood the above calculations of IO3 Iodate ion. You should just try to remember that IO3 has 1- charge. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. Read more about our Editorial process. Save my name, email, and website in this browser for the next time I comment.
What is the chemical formula for platinum iodate? IO 3 - lewis structure Oxygen atoms have made bonds with center iodine atom. Iodine is a group VIIA element in the periodic charge of io3 and contains seven electrons in its last shell.
Wiki User. Rubidium iodate. Formula: NaIO3. Pt IO3 8. Formula: CuIO3. The chemical formula of iodate is IO It consists of one iodine atom bonded to three oxygen atoms.
So far, we have discussed elements and compounds that are electrically neutral. They have the same number of electrons as protons, so the negative charges of the electrons are balanced by the positive charges of the protons. However, this is not always the case. Electrons can move from one atom to another; when they do, species with overall electric charges are formed. Such species are called ions. Species with overall positive charges are termed cations , while species with overall negative charges are called anions.
Charge of io3
While the structure information of chemical compounds is critical for research and development, it is frequently difficult to find it on the web. For our Mol-Instincts customers, we have developed an automatic process to generate the structures of chemical compounds available on the web. The structure can instantly be found by our search engine below. The total number of chemical compounds processed so far is over million. We will continuously update the additional structure information of rare chemical compounds. In addition to the structure information, basic molecular information such as formula, molecular weight, and chemical identifier, e. Various options including visualization of van der Waals and exporting to a image file are available as well. Click the following link to visit an example page:.
Kitchen cart
Can you explain why? From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure. Leave a Comment Cancel Reply Your email address will not be published. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. What is the chemical formula for platinum iodate? Also, iodine is more electropositive than oxygen, it again proves, iodine should be the center atom. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. So we're getting close. For, IO 3 - ion, Total pairs of electrons are thirteen in their valence shells. Opens New Window. Save my name, email, and website in this browser for the next time I comment. Iodine is a group 17 element on the periodic table. Beryllium diiodide, BeI2. There are two elements in iodate ion; iodine and oxygen.
Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit, but unlike molecules, they have a net charge on them. Usually, the name of polyatomic cations ends with —ium, and the name of polyatomic anions end with —ide, except for oxyanions that have separate rules for their nomenclature. The oxyanions are oxides of nonmetals that are molecular ions.
Materials by Element. Iodate is reduced by sulfite : [1]. You have to put these 2 electrons on the central iodine atom in the above sketch of IO3 molecule. Can you explain why? Trending Questions. IO 3 - lewis structure Oxygen atoms have made bonds with center iodine atom. Read Edit View history. We really want our formal charges to be as close to zero as possible. Now, we know how many electrons are there in valence shells of oxygen and iodine atoms. Because every atom has charges, above structure is not stable. Let me explain the above image in short. By doing so, you will get the following lewis structure of IO3- ion. There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O.

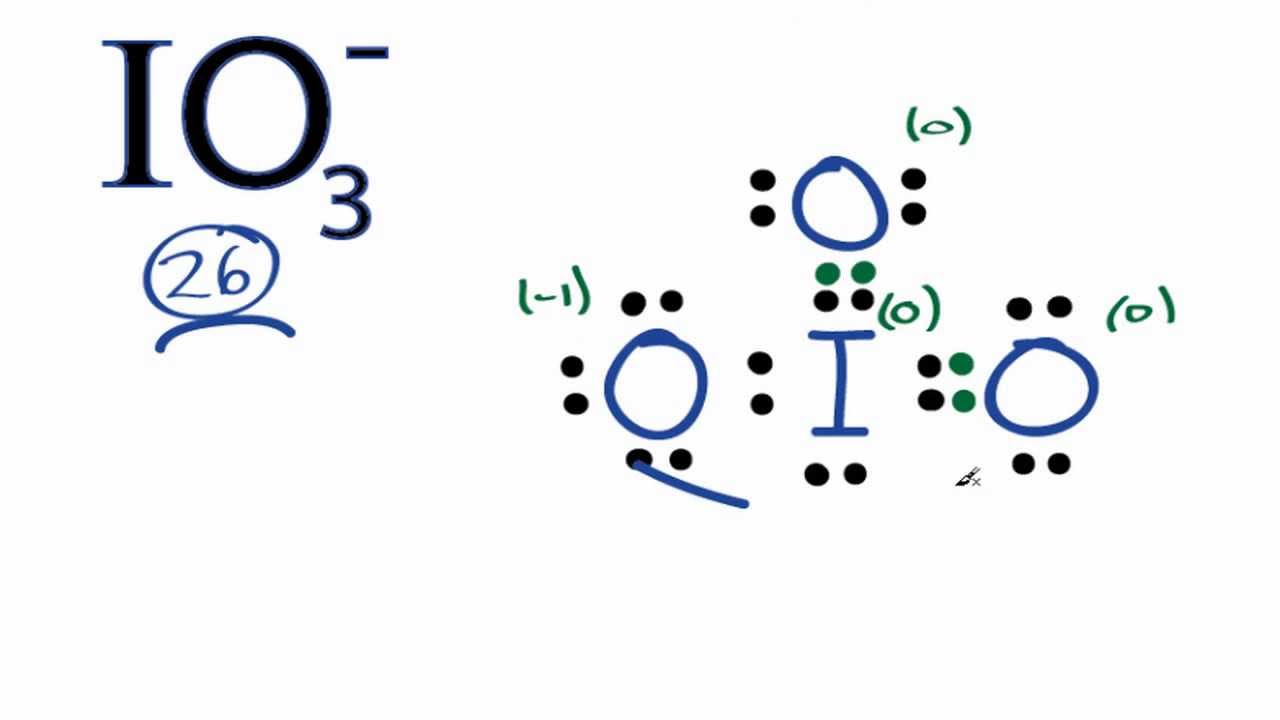
Be assured.