Daniell cell class 12
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell daniell cell class 12 are in use as the anode and cathoderespectively. Both metals are submerged in the corresponding salt solutions.
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments. I would like to thank my parents for supporting my work on this project. One of their oldest and most simple incarnation was the Daniell cell. If u have ever wondered how a cell works, the Daniell cell is the best way to practically experience and understand it.
Daniell cell class 12
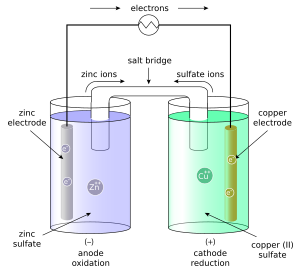
The Daniell cell is a type of electrochemical cell invented in by John Frederic Daniell , a British chemist and meteorologist , and consists of a copper pot filled with a copper II sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile , and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the s by a Frenchman named Callaud and became a popular choice for electrical telegraphy. The Daniell cell is also the historical basis for the contemporary definition of the volt , which is the unit of electromotive force in the International System of Units. The definitions of electrical units that were proposed at the International Conference of Electricians were designed so that the electromotive force of the Daniell cell would be about 1. In the Daniell cell, copper and zinc electrodes are immersed in a solution of copper II sulfate and zinc sulfate , respectively. At the anode negative electrode , zinc is oxidized as per the following half reaction:. At the cathode positive electrode , copper is reduced as per the following reaction:. Note that positively charged copper ions move towards the positive electrode, driven by a reduction in chemical energy. These processes result in the accumulation of solid copper at the cathode and the corrosion of the zinc electrode into the solution as zinc cations. In classroom demonstrations, a form of the Daniell cell known as two half cells is often used due to its simplicity. The two half cells each support one half of the reactions described above.
The Daniell cell was invented, while the chemist was seeking a way to eliminate the hydrogen bubble issue found in the voltaic pile.
How does a Cell in a T. V remote make it work or how a Battery of Mobile Phone Charges when connected to its charger? All such questions are answered in the branch of Science known as Electrochemistry. Electrochemistry is the study of producing Electricity through Chemical reactions and also the use of Electricity to carry out non-spontaneous Chemical reactions. To achieve the above-mentioned aim Cells are used.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell. The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate. Register Now. A conventional galvanic cell, it is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions. A copper vessel makes up this cell. In this case, a saturated CuSO 4 solution is used as a depolarizer and diluent. Fill with H 2 SO 4 , which works as an electrolyte. Zn 2 SO 4 is used to submerge a zinc rod that has been amalgamated.
Daniell cell class 12
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions. A Daniell cell is a device that transforms chemical energy released by redox reactions into electrical energy. It has a 1. Zinc Zn , which serves as the anode in a Daniell Cell, and Copper Cu , which serves as the cathode, are the two different metals in use. Each of these electrodes continues to be immersed in ion-based chemical solutions. In the Daniell cell, zinc dissolves in zinc sulphate and copper dissolves in copper II sulphate. Cell electrodes are metal strips.
سكس دكتور مصري
These Cells are those Cells that produce Electricity through Chemical reactions. This reduces the internal resistance of the system and thus the battery yields a stronger current. Is a Daniell cell reversible? Daniell Cells are used in Battery development and Electrical telegraphy. In an electrochemical cell, the anode is negative and the cathode is positive. This container is porous and contains dilute sulphuric acid. Copper sulfate crystals are scattered around the cathode and the jar then filled with distilled water. Galvanic, also known as Voltaic, and electrolytic cells are the two different types of electrochemical cells. Chem Ip Chem Ip. The anode is positive, the cathode is negative. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. In classroom demonstrations, a form of the Daniell cell known as two half cells is often used due to its simplicity. The copper electrode plats out copper ions from the copper II sulphate solution while the zinc electrode is consumed. How does Daniell cell work? Cells are devices in which Chemical Reactions due to Electricity or produces Electricity.
How does a Cell in a T.
As we have seen above, the setup of the Daniell Cell and the Cell reactions taking place in the Daniell Cell now we can understand the working of Daniell Cells. Professional Documents. It prolongs the life of the cell. Since neither half reaction will occur independently of the other, the two half cells must be connected in a way that will allow ions to move freely between them. When the zinc and copper electrodes are joined by wire, the following observations are made: There is a flow of electric current through the external circuit. The concentration of ZnSO4 solution increases while the concentration of copper sulphate solution decreases. The left part is oxidation half Cell and the right part is reduction half Cell. A voltaic cell can be either reversible or irreversible, whereas the Daniell cell is reversible. Give half cell equation of Daniel cell takes place at cathode. Welcome onboard. Studying the tough portion first may feel like a burden initially but day by day, it will help you gain confidence and acquire the subject with more positivity. FREE Signup.

0 thoughts on “Daniell cell class 12”