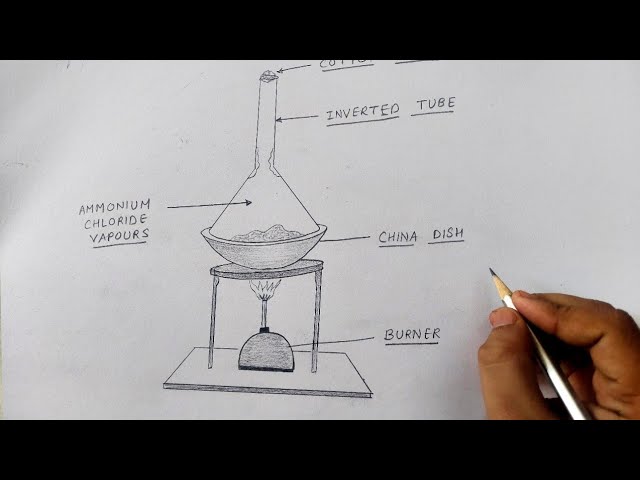
Diagram of sublimation of ammonium chloride
Ammonium chloride when heated decomposes into hydrogen chloride and ammonia.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Diagram of sublimation of ammonium chloride
Sign in Open App. Write the help of a labelled diagram describe in brief and activity to show sublimation of ammonium chloride? Most Upvoted Answer. Here is a brief description of the diagram: 1. Procedure: - Place a small amount of ammonium chloride in the round-bottomed flask. Labelled Diagram: The labelled diagram should clearly show the different components of the apparatus and the direction of gas flow. It should also indicate the heating source and the cooling arrangement. Place a small quantity of ammonium chloride in the test tube. Attach the glass funnel to the mouth of the test tube, ensuring a tight seal. Place the test tube in a beaker containing cold water or ice. Heat the bottom of the test tube using a Bunsen burner flame. Observations: As the ammonium chloride is heated, it gradually sublimes, meaning it directly changes from a solid state to a gaseous state without passing through the liquid phase. The sublimation process is indicated by the formation of white fumes or a white solid deposit on the cooler parts of the test tube or the funnel.
What is the correct order of the methods you would apply to separate the components of a mixture of ammonium chloride, common salt and sand?
With the help of a labelled diagram, describe the method of separating ammonium chloride from a mixture of ammonium chloride and common salt. Mention the difference in the properties of ammonium chloride and sodium chloride which has made this separation possible. Byju's Answer. Open in App. There are many substances that are converted into a gas from a solid when heated and converted from gas to solid when cooled without converting into liquid.
Some compounds are capable of sublimation, which is the direct phase change from solid to gas. Solid carbon dioxide is an example of a substance that sublimes readily at atmospheric pressure, as a chunk of dry ice will not melt, but will seem to "disappear" as it turns directly into carbon dioxide gas. Sublimation is an analogous process to boiling, as it occurs when a compound's vapor pressure equals its applied pressure often the atmospheric pressure. The difference is that sublimation involves a solid's vapor pressure instead of a liquid's. Most solids do not have an appreciable vapor pressure at easily accessible temperatures, and for this reason the ability to sublime is uncommon. Compounds that are capable of sublimation tend to be those with weak intermolecular forces in the solid state. These include compounds with symmetrical or spherical structures. Examples of compounds that can be sublimed are in Figure 6. As relatively few solids are capable of sublimation, the process can be an excellent purification method when a volatile solid is contaminated with non-volatile impurities.
Diagram of sublimation of ammonium chloride
Place the sample to be sublimed in the bottom of the sublimation apparatus. Be sure to apply the vacuum first, then coolant. If cooled before the vacuum, condensation may occur on the cold finger. Delicately reinstate the air pressure, noting that an abrupt opening of the vessel will cause air to knock crystals off the cold finger. Search site Search Search. Go back to previous article. Sign in. Figure 6. Spread the crude, dry solid to be sublimed in a thin layer on a "bottom" petri dish Figure 6.
Yeti bcd
Data, Monograph 9 , , Chapter Notes For Class 9. Eustis, Whiteside, et al. Explore Courses for Class 9 exam. Experiment of sublimation of ammonium chloride. Follow Us. Evlasheva, Potapov, et al. Kafafi B - John E. Similar Class 9 Doubts With the help of labelled diagram describe in brief an activity to show sublimation of Ammonium Chloride? Signup now for free. Edu cation Rev olution. Anuj Dutt answered this. Enter OTP.
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Notable examples include sublimation of dry ice at room temperature and atmospheric pressure, and that of solid iodine with heating. The reverse process of sublimation is deposition also called desublimation , in which a substance passes directly from a gas to a solid phase, without passing through the liquid state.
Note: Please consider using the reaction search for this species. Here you can find the meaning of Write the help of a labelled diagram describe in brief and activity to show sublimation of ammonium chloride? View All Courses. Learn this topic in detail More from Related Course. Acree, Jr. It should also indicate the heating source and the cooling arrangement. Name two substances other than ammonium chloride which undergo sublimation. Join with a free account. Which of the following mixture can be separated completely by the process of sublimation? What is the meaning of process of sublimation Name any two solid substances whose mixture can be separated by sublimation. Create you account for free.

I congratulate, what words..., a magnificent idea
The charming answer
I am assured, that you have deceived.