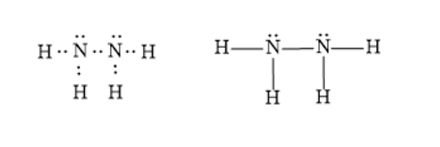
Dinitrogen tetrahydride
Submitted by Parker P. Your personal AI tutor, companion, and study partner.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago. NCl3 nitrogen trichloride b. BCl3 boron trichloride c.
Dinitrogen tetrahydride
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, the processes are energy-intensive. Is it possible to discover a homogeneous catalyst that can convert molecular nitrogen into higher-value organonitrogen compounds using a less energy-intensive pathway? If this could be achieved, it would be considered a major breakthrough in this area. In contrast to carbon monoxide, which is reactive and an important feedstock in many homogeneous catalytic reactions, the ischelectronic but inert N-2 molecule is a very poor ligand and not a common industrial feedstock, except for the above-mentioned industrial production of NH3. Because N-2 is readily available from the atmosphere and because nitrogen is an essential element for the biosphere, attempts to discover new processes involving this simple small molecule have occupied chemists for over a century. Since the first discovery of a dinitrogen complex in , inorganic chemists have been key players in this area and have contributed much fundamental knowledge on structures, binding modes, and reactivity patterns. For the most part, the synthesis of dinitrogen complexes relies on the use of reducing agents to generate an electron-rich intermediate that can interact with this rather inert molecule. In this Account, a facile reaction of dinitrogen with a ditantalum tetrahydride species to generate the unusual side-on end-on bound N-2 moiety is described.
In other projects.
E-mail: ynishiba sogo. A dinitrogen-bridged dimolybdenum-tetrachloride complex is prepared and reduced with Super-Hydride LiBHEt 3 to afford the corresponding dimolybdenum-dinitrogen complex together with the formation of molecular dihydrogen. This reaction proceeds via the ligand exchange of the coordinated dihydrogen generated in situ with molecular dinitrogen. As the next stage of the previous work, we have focused on the development of the catalytic formation of ammonia from molecular dinitrogen and dihydrogen at ambient temperature and pressure. To achieve the catalytic formation of ammonia as the next nitrogen fixation, in place of the Haber—Bosch process, 7 the ruthenium—hydride species should reduce the high oxidative tungsten species to regenerate the corresponding tungsten—dinitrogen complex. However, unfortunately, the tungsten species can not be reduced with the ruthenium—hydride species or with other hydride species such as LiBHEt 3.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms the elements of group 14 are carbon , silicon , germanium , tin , lead and flerovium. The tetrahydride series has the chemical formula XH 4 , with X representing any of the carbon family. Methane is commonly the result of the decomposition of organic matter and is a greenhouse gas. The other hydrides are generally unstable, poisonous metal hydrides. They take on a pyramidal structure, and as such are not polar molecules like the other p-block hydrides.
Dinitrogen tetrahydride
Hydrazine is a molecule of two singly-bonded nitrogen atoms and four peripheral hydrogen atoms. In its anhydrous form, it is a colourless, toxic irritant and sensitiser, which damages the central nervous system, producing symptoms as extreme as tumours and seizures. The pungent smell of hydrazine is not unlike that of ammonia, and it is so powerful a reducing agent that it is highly explosive. Considering this, it seems strange that around , metric tonnes of the stuff are manufactured worldwide every year. But hydrazine does influence our everyday lives. It keeps us warm, clothes and feeds us, can save our lives and even take us to the moon.
Manual awning
Pedro Paulet, sabio multidisciplinario in Spanish. So from here we have to tell the balance situation for this. It is a hypergolic propellant in combination with a hydrazine -based rocket fuel. Is there any difference? Which of these represents a properly balanced equation for this reaction? Dinitrogen tetrahydride reacts with dinitrogen tetraoxide to form nitrogen gas and water. This problem has been solved! Precautionary statements. Solar Energy. Scheme 5 Oxidation of 3 with AgCl to form 2. Presentation on theme: "dinitrogen tetrahydride"— Presentation transcript:. CCDC Such dissociative gas Brayton cycles have the potential to considerably increase efficiencies of power conversion equipment.
This chapter describes the activation of dinitrogen by various transition metal hydride complexes. A number of mononuclear transition metal hydride complexes can incorporate dinitrogen, but they are usually difficult to induce N—N bond cleavage. In contrast, multimetallic hydride complexes can split and hydrogenate dinitrogen through cooperation of the multiple metal hydrides.
Wang Lawndale High School. This species reacts with water to give both nitrous acid and nitric acid :. Join Numerade as a. New York: Reinhold. Chemical formula. Many of the anhydrous transition metal nitrates have striking colours. Descriptive inorganic chemistry 6th ed. Infobox references. In this Account, a facile reaction of dinitrogen with a ditantalum tetrahydride species to generate the unusual side-on end-on bound N-2 moiety is described. Upper Saddle River, N.

Excuse for that I interfere � To me this situation is familiar. I invite to discussion. Write here or in PM.
It is remarkable, it is the amusing information