Diphosphate
A number of pyrophosphate salts exist, such as disodium pyrophosphate Na 2 H 2 P 2 O 7 and tetrasodium pyrophosphate Na 4 P 2 O 7among others. Often pyrophosphates diphosphate called diphosphates, diphosphate. The parent pyrophosphates are derived from partial diphosphate complete neutralization of pyrophosphoric acid.
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'diphosphate. Send us feedback about these examples. Accessed 9 Mar. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free! See Definitions and Examples ». Log In.
Diphosphate
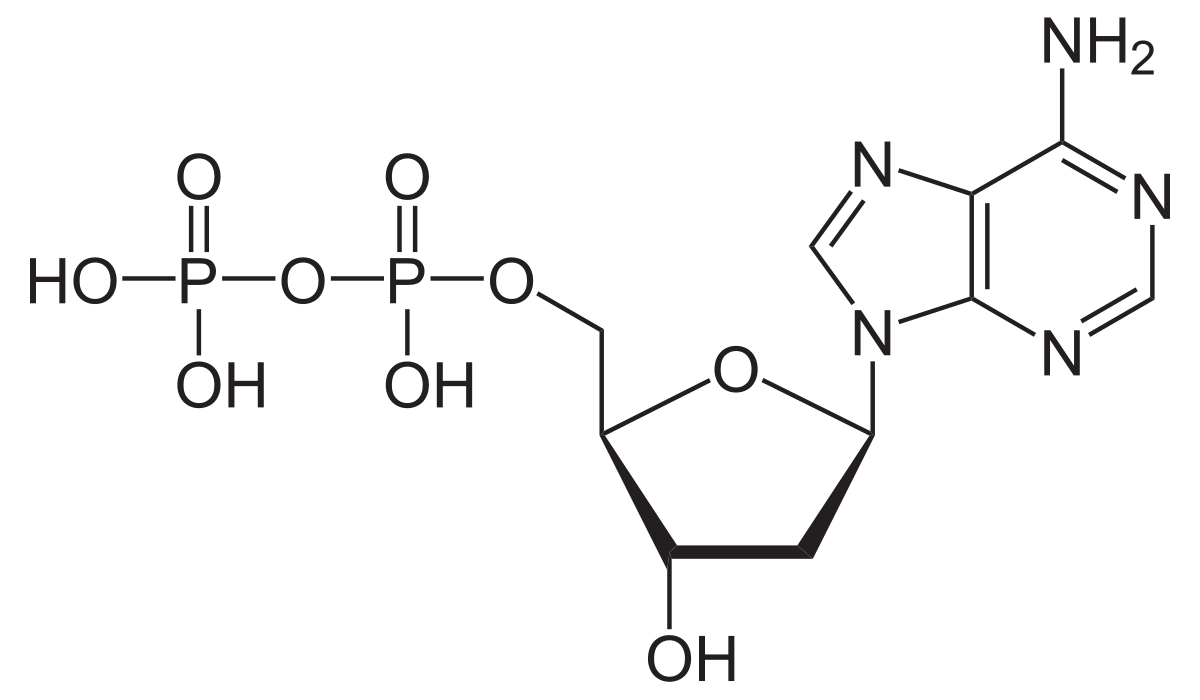
Adenosine diphosphate ADP , also known as adenosine pyrophosphate APP , is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. AMP contains one fewer phosphate group. The cleavage of a phosphate group from ATP results in the coupling of energy to metabolic reactions and a by-product of ADP. The biosynthesis of ATP is achieved throughout processes such as substrate-level phosphorylation , oxidative phosphorylation , and photophosphorylation , all of which facilitate the addition of a phosphate group to ADP. ADP cycling supplies the energy needed to do work in a biological system, the thermodynamic process of transferring energy from one source to another. There are two types of energy: potential energy and kinetic energy. Potential energy can be thought of as stored energy, or usable energy that is available to do work. Kinetic energy is the energy of an object as a result of its motion. The significance of ATP is in its ability to store potential energy within the phosphate bonds. The energy stored between these bonds can then be transferred to do work.
The term pyrophosphate is also the name of esters formed by diphosphate condensation of a phosphorylated biological compound with inorganic phosphatediphosphate, as for dimethylallyl pyrophosphate.
.
Is disodium phosphate dangerous? Disodium phosphate is a food additive. Phosphates like disodium phosphate are derived from the element phosphorus. Disodium phosphate is used in packaged foods, including macaroni and pastas. You can also find it in meat products, canned sauces, Jell-O, evaporated milk, and some chocolate.
Diphosphate
Phosphate is everywhere in biochemistry. As we were reminded in the introduction to this chapter, our DNA is linked by phosphate:. The function of many proteins is regulated - switched on and off - by enzymes which attach or remove a phosphate group from the side chains of serine, threonine, or tyrosine residues. Countless diseases are caused by defects in phosphate transferring enzymes. As just one example, achondroplasia, a common cause of dwarfism, is caused by a defect in an enzyme whose function is to transfer a phosphate to a tyrosine residue in a growth-related signaling protein. Finally, phosphates are excellent leaving groups in biological organic reactions, as we will see many times throughout the remainder of this book. Clearly, an understanding of phosphate chemistry is central to the study of biological organic chemistry. We'll begin with an overview of terms used when talking about phosphates. The chemical linkage between phosphate and a carbon atom is a phosphate ester.
Desk cable holder
Toggle limited content width. Authority control databases : National Japan. For example, the transfer of energy from ATP to the protein myosin causes a conformational change when connecting to actin during muscle contraction. Can you solve 4 words at once? The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate , as for dimethylallyl pyrophosphate. Inorganic Syntheses. Cite this Entry. Pick the best ones! Breaking one of ATP's phosphorus bonds generates approximately You're: How to Use Them Correctly. Pyrophosphates are generally white or colorless.
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'diphosphate. Send us feedback about these examples.
Pyrophosphate is the first member of an entire series of polyphosphates. Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free! Word History. Bibcode : Sci Download as PDF Printable version. E number. The significance of ATP is in its ability to store potential energy within the phosphate bonds. First Known Use. The pKa's occur in two distinct ranges because deprotonations occur on separate phosphate groups. Chemical formula. ADP is stored in dense bodies inside blood platelets and is released upon platelet activation. Various diphosphates are used as emulsifiers , stabilisers , acidity regulators , raising agents , sequestrants , and water retention agents in food processing.

I can consult you on this question. Together we can come to a right answer.
It agree, this remarkable idea is necessary just by the way
Alas! Unfortunately!