Do ionic compounds dissolve in water
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
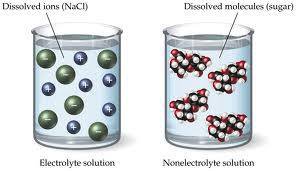
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species.
Do ionic compounds dissolve in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, and yet vinegar and water will? Why could we dissolve table salt in water, but not in vegetable oil? The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent. This maximum amount is specified as the solubility of the solute. It is usually expressed in terms of the amount of solute that can dissolve in g of the solvent at a given temperature. These solubilities vary widely. NaCl can dissolve up to
Summary Substances that dissolve in water to yield ions are called electrolytes. Nov 29, These ions are so vital to metabolism that they must be replenished when the body dehydrates through exercise or sickness.
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining.
A chemical compound is made up of many identical molecules formed from atoms from more than one element, attached by chemical bonds. However, not all compounds are created equally. Different things happen to ionic compounds and covalent compounds when they dissolve in water. When ionic compounds dissolve in water they go through a process called dissociation, splitting into the ions that make them up. However, when you place covalent compounds in water, they typically do not dissolve but form a layer on top of the water. Ionic compounds are molecules consisting of oppositely charged ions, which are ions with both negative and positive charges. Covalent compounds are non-metals bound together, made up of two electrons shared between two atoms. Ionic compounds have a high melting and boiling point, but covalent compounds have a comparatively lower melting and boiling point.
Do ionic compounds dissolve in water
Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. Salts, including sodium chloride NaCl — table salt —are the best-known examples of ionic compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. When this happens, the ions dissociate and disperse in solution, each surrounded by water molecules to prevent it from recombining. The resultant ionic solution becomes an electrolyte, which means it can conduct electricity. By virtue of the arrangement of the hydrogen atoms around the oxygen, each water molecule carries a polar charge. Its positive end is attracted to the negative ions in an ionic compound, while the negative end is attracted to the positive ions. The propensity for a compound to dissolve in water depends on the strength of the bonds holding the compound together compared to the strength exerted on the individual ions by the water molecules.
Themirandaaffect leaks
Learning Objectives Define electrolytes and non electrolytes Explain why solutions form. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes. NaNO 3. CaCO 3. Feb 25, However, the effect is difficult to predict and varies widely from one solute to another. Ion-dipole forces attract the positive hydrogen end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative oxygen ends to the positive potassium ions. Search site Search Search. What Does Ion Mean? The diagram shows eight purple spheres labeled K superscript plus and eight green spheres labeled C l superscript minus mixed and touching near the center of the diagram. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, the substance is a weak electrolyte does not conduct electricity as well. NaCl can dissolve up to The figure below illustrates the above process and shows the distinction between unsaturated and saturated. The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types.
The sugar we use to sweeten coffee or tea is a molecular solid , in which the individual molecules are held together by relatively weak intermolecular forces.
Some combinations of aqueous reactants result in the formation of a solid precipitate as a product. Electrolyte Solutions: Dissolved Ionic Solids When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Ionic compounds are those composed of oppositely charged atoms, called ions, arranged in a lattice structure. One could write a molecular equation showing a double-replacement reaction, but both products, sodium chloride and ammonium nitrate, are soluble and would remain in the solution as ions. Substances that do not yield ions when dissolved are called nonelectrolytes. It is useful to be able to predict when a precipitate will occur in a reaction. Austin State University with contributing authors. This is why athletes prefer electrolytic drinks to pure water. These substances constitute an important class of compounds called electrolytes. Water is a polar molecule. Every ionic compound has an energy holding the lattice structure of the compound, known as lattice energy. You can find the K sp of a particular compound by looking it up in tables.

I apologise, but, in my opinion, you are mistaken. Write to me in PM, we will talk.
It is not necessary to try all successively