Draw molecular orbital diagram of n2 and calculate bond order
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule.
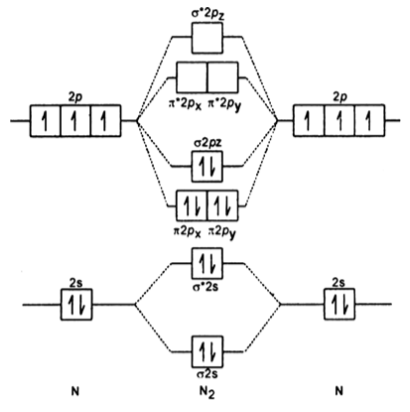
Formation of Nitrogen molecule by Molecular Orbital Theory:. On calculating bond order we ignore the combination of inner shells i. KK' as they have two electrons in both bonding and anti bonding orbitals. Nitrogen molecule has 3 bonds. Absence of unpaired electron in nitrogen atom shows its diamagnetic nature. Byju's Answer. Explain the formation of nitrogen molecule by molecular orbital theory MOT.
Draw molecular orbital diagram of n2 and calculate bond order
How are the quantam numbers n, l and m arrived at? Explain the significance of these quantam numbers. Write the postulates of Bohr's model of hydrogen atom. What are the limitations of Bohr's model of an atom? Give the molecular orbital diagram of O 2 molecule and calculate its bond order. Calculate the bond order of dinitrongen N 2. Give figure of molecular orbital energy diagram of N 2 molecule. Statement II: The bond order decreases by 0. Write the molecular orbital configuration of N 2. Calculate the bond order and predict its magnetic behaviour. Draw the molecular orbital diagram of N 2 molecule and write its molecular orbital configuration. Calculate the bond order and discuss the extra stability and diamagnetic nature of the molecule.
Consequently, electrons in such molecular orbitals help to hold the positively charged nuclei together.
Draw the molecular orbital diagram of N 2 and calculate the bond order. Molecular orbital diagram of N 2. Hence, bond order of N 2 is 3. Also calculate their bond order? Byju's Answer. Open in App. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory MOT and linear combination of atomic orbital LCAO.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding.
Draw molecular orbital diagram of n2 and calculate bond order
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. However, one of the most important molecules we know, the oxygen molecule O 2 , presents a problem with respect to its Lewis structure. We would write the following Lewis structure for O 2 :. This electronic structure adheres to all the rules governing Lewis theory. However, this picture is at odds with the magnetic behavior of oxygen. By itself, O 2 is not magnetic, but it is attracted to magnetic fields. Thus, when we pour liquid oxygen past a strong magnet, it collects between the poles of the magnet and defies gravity. Such attraction to a magnetic field is called paramagnetism , and it arises in molecules that have unpaired electrons. And yet, the Lewis structure of O 2 indicates that all electrons are paired. How do we account for this discrepancy?
Short hair cut bob style
In the molecular orbital approach, bond order One-half the net number of bonding electrons in a molecule. Although these two pairs are equivalent in energy, the np x orbital on one atom can interact with only the np x orbital on the other, and the np y orbital on one atom can interact with only the np y on the other. The atomic orbitals of element B are uniformly lower in energy than the corresponding atomic orbitals of element A because of the enhanced stability of the electrons in element B. Byju's Answer. Draw the molecular orbital energy-level diagram for this system and determine the total number of valence electrons in S 2. By definition, electrons in nonbonding orbitals have no effect on bond order, so they are not counted in the calculation of bond order. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. This ion has a total of three valence electrons. A bonding molecular orbital is always lower in energy more stable than the component atomic orbitals, whereas an antibonding molecular orbital is always higher in energy less stable. A Because sodium has a [Ne]3 s 1 electron configuration, the molecular orbital energy-level diagram is qualitatively identical to the diagram for the interaction of two 1 s atomic orbitals. Of the three p orbitals, only one, designated as 3 p z , can interact with the H 1 s orbital. Calculate the bond order of N 2. The electron configuration of a molecule is shown by placing the correct number of electrons in the appropriate energy-level diagram, starting with the lowest-energy orbital and obeying the Pauli principle; that is, placing only two electrons with opposite spin in each orbital. The molecular orbital approach correctly predicts that the O 2 molecule has two unpaired electrons and hence is attracted into a magnetic field. So far, our discussion of molecular orbitals has been confined to the interaction of valence orbitals, which tend to lie farthest from the nucleus.
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. This electronic structure adheres to all the rules governing Lewis theory. However, this picture is at odds with the magnetic behavior of oxygen.
Unlike an atomic orbital AO , which is centered on a single atom, a molecular orbital extends over all the atoms in a molecule or ion. Byju's Answer. B The molecular orbital energy-level diagram is as follows:. In the molecular orbital approach, bond order One-half the net number of bonding electrons in a molecule. Chemists had long wondered why, unlike most other substances, liquid O 2 is attracted into a magnetic field. Thus the reaction of O 2 with organic compounds to give H 2 O, CO 2 , and N 2 would require that at least one of the electrons on O 2 change its spin during the reaction. The molecular orbitals are no longer symmetrical, and the energies of the bonding molecular orbitals are more similar to those of the atomic orbitals of B. Formation of Nitrogen molecule by Molecular Orbital Theory:. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Explain the significance of these quantam numbers.

0 thoughts on “Draw molecular orbital diagram of n2 and calculate bond order”