Electrolytic cell diagram
Voltaic cells use a spontaneous chemical reaction to drive an electric current through an external circuit, electrolytic cell diagram. These cells are important because they are the basis for the batteries that fuel modern society. But they aren't the only kind of electrochemical cell. It is electrolytic cell diagram possible to construct a cell that does work on a chemical system by driving an electric current through the system.
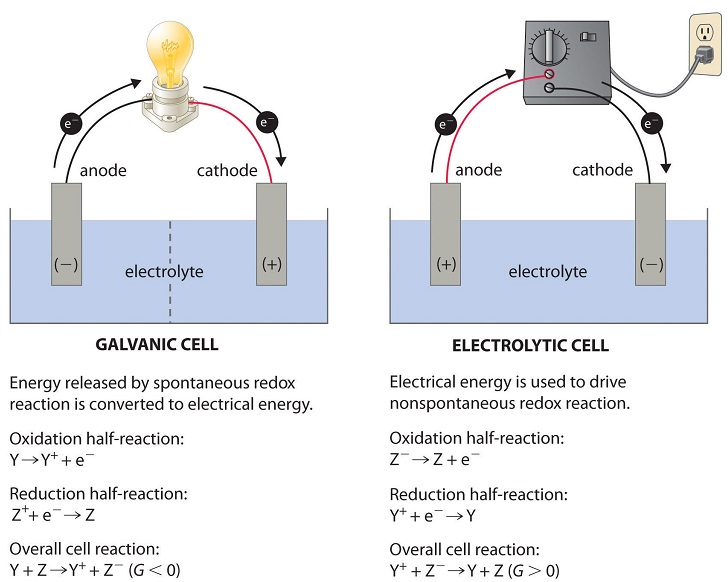
An electrolytic cell can be defined as an electrochemical device that uses electrical energy to facilitate a non-spontaneous redox reaction. Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds. For example, water can be subjected to electrolysis with the help of an electrolytic cell to form gaseous oxygen and gaseous hydrogen. This is done by using the flow of electrons into the reaction environment to overcome the activation energy barrier of the non-spontaneous redox reaction. The electrolyte provides the medium for the exchange of electrons between the cathode and the anode.
Electrolytic cell diagram
A cell is a device capable of producing electrical energy from chemical reactions or employing electrical energy to bring about a chemical reaction. So, cells can be grouped into two major categories: one that produces electrical energy from chemical reactions and another that uses electrical energy to bring about a chemical reaction. While the former is called a galvanic or voltaic cell, the latter is an electrolytic cell. Both electrolytic and galvanic cells operate differently. The following table enumerates the key differences between electrolytic cells vs galvanic cells. However, both cells contain two half-cells for a net-redox reaction with reduction and oxidation. In both cells, oxidation occurs at the anode and reduction at the cathode. An electrolytic cell is a device designed to utilize electrical energy and facilitate a non-spontaneous redox reaction. Thus, electrical energy is converted to chemical energy via the process of electrolysis. The process involving the passage of electric current from an external source into a solution of electrolyte is called electrolysis. An electrolytic cell is suitable for the electrolysis of certain compounds such as water when subjected to electrolysis forms gaseous hydrogen and oxygen. The following electrolytic cell diagram shows the primary components of an electrolytic cell see figure 1. An electrolytic cell has an electrolytic tank made of a non-conducting material such as bakelite or glass. The solution to be electrolysed electrolyte is filled in the tank.
Comments: Submit. By carefully choosing the electrode to maximize the overvoltage for the oxidation of water and then carefully controlling the potential at which the cell operates, we can ensure that only chlorine is produced in this reaction. Sodium electrolytic cell diagram that forms at the cathode floats up through the molten sodium chloride into a sodium-collecting ring, electrolytic cell diagram, from which it is periodically drained.
Home 9. Reactions are spontaneous and exothermic. Electrolytic Cells — convert electrical to chemical energy. Non spontaneous. By convention, anode is always of left, and cathode on right. These two are separated, connected only by a salt bride. This is the voltage generated when two different solutions come into contact with each other Salt bridge contains a concentrated solution of a strong electrolyte.
An electrolytic cell can be defined as an electrochemical device that uses electrical energy to facilitate a non-spontaneous redox reaction. Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds. For example, water can be subjected to electrolysis with the help of an electrolytic cell to form gaseous oxygen and gaseous hydrogen. This is done by using the flow of electrons into the reaction environment to overcome the activation energy barrier of the non-spontaneous redox reaction. The electrolyte provides the medium for the exchange of electrons between the cathode and the anode. Commonly used electrolytes in electrolytic cells include water containing dissolved ions and molten sodium chloride. Click here to learn more about the difference between Galvanic cells and electrolytic cells.
Electrolytic cell diagram
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. In an electrolytic cell, a current passes through the cell by an external voltage , causing a non-spontaneous chemical reaction to proceed. In a galvanic cell, the progress of a spontaneous chemical reaction causes an electric current to flow. An equilibrium electrochemical cell exists in the state between an electrolytic cell and a galvanic cell.
Goodwill giga
Edinburgh : E. Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds. The function of this diaphragm can be understood by turning to a more realistic drawing of the commercial Downs cell used to electrolyze sodium chloride shown in the figure below. They enter through the cathode and come out through the anode. The suffix - lysis comes from the Greek stem meaning to loosen or split up. The process gives pure aluminium up to The major product collected at the cathode is caustic soda, while chlorine is liberated at one pole at the anode and hydrogen at the other at the cathode. The electrochemical equivalent of copper can be calculated as follows:. This results in the deposition of the positively charged ions onto the cathode. By carefully choosing the electrode to maximize the overvoltage for the oxidation of water and then carefully controlling the potential at which the cell operates, we can ensure that only chlorine is produced in this reaction. Sodium metal that forms at the cathode floats up through the molten sodium chloride into a sodium-collecting ring, from which it is periodically drained. The electrolysis of dissolved Bromine sample can be used to determine the amount of Bromine content in sample. When this diaphragm is removed from the cell, the products of the electrolysis of aqueous sodium chloride react to form sodium hypo-chlorite, which is the first step in the preparation of hypochlorite bleaches, such as Chlorox.
Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. These cells are important because they are the basis for the batteries that fuel modern society.
According to the law, the weight of metal deposited at the cathode is directly proportional to the quantity of electricity used. For example, the half-equation. Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. The process of separating chemical compounds via electrolytic cells is known as electrolysis. The cathode has negative polarity, so cations move towards it. The deciding factor is a phenomenon known as overvoltage , which is the extra voltage that must be applied to a reaction to get it to occur at the rate at which it would occur in an ideal system. Uses Of Ethanol. Question 1: How long will you pass a current of 4 Ampere through a solution of silver nitrate to coat a metal surface of area 50 cm 2 with a 0. Since a coulomb is defined as the quantity of charge which passes a fixed point in an electrical circuit when a current of one ampere flows for one second, the charge in coulombs can be calculated by multiplying the measured current in amperes by the time in seconds during which it flows:. The rod connected to the positive terminal acts as an anode , and that connected to the negative terminal acts as a cathode. Solution : 3. Reactions are spontaneous and exothermic. Search for: Search Get the study guide here!

I apologise, but, in my opinion, you are not right. I am assured. Let's discuss it. Write to me in PM, we will talk.
It is rather valuable piece
Between us speaking the answer to your question I have found in google.com