Electron dot structure of ch3cl
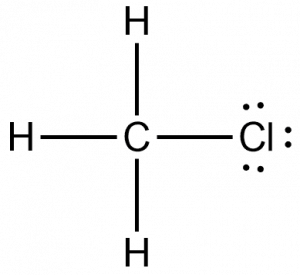
Chloromethane CH 3 Cl contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom.
There are 3 lone pairs on the Chlorine atom Cl. In order to find the total valence electrons in a CH3Cl molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Chlorine is group 17 element on the periodic table.
Electron dot structure of ch3cl
The chloromethane chemical formula is CH3Cl. The carbon, chlorine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, chlorine, and hydrogen are four, seven, and one respectively. Chloromethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Cl Lewis structure can be used. The first step is to sketch the Lewis structure of the CH3Cl molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the one chlorine and three hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH3Cl Lewis Structure. Finally, you must add their bond polarities to compute the strength of the C-Cl bond dipole moment properties of the CH3Cl molecule. The CH3Cl molecule is classified as a polar molecule. The molecule of chloromethane with tetrahedral molecular geometry is tilted, the bond angles between chlorine, carbon, and hydrogen are As a result, it has the permanent dipole moment. The CH3Cl molecule has a permanent dipole moment due to an unequal charge distribution of negative and positive charges. The net dipole moment of the CH3Cl molecule is 1.
The C-Cl and C-H bond lengths are and pm picometer respectively. The chlorine core terminal one single bond connected with the central carbon atom of the CH3Cl molecule has seven valence electrons, six lone pairs of electrons, and two bonding electrons.
.
Chloromethane or Methyl chloride having a molecular formula of CH 3 Cl is an organic compound. It is an odorless and transparent gas that was initially used as a refrigerant. Later it was found that this gas is toxic and can harm the central nervous system of humans. Although it is no longer used as a refrigerant, Chloromethane has many uses and applications in several chemical and pharmaceutical industries. To understand its chemical properties and physical properties, one needs first to know the Lewis structure and molecular geometry of CH 3 Cl. And to help you with understanding its structure in-depth, I will help you to make its Lewis structure step-by-step in this blog post. But before looking at that, let us first discuss the valence electrons present in this compound as these electrons are the ones that form bonds. This happens because it tries to achieve the same valence electron configuration as inert gases. The atoms that have complete octets or rather suffice the octet rule become inert and non-reactive.
Electron dot structure of ch3cl
Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Interestingly, it has been studied by the researchers that the average life of chloromethane in the air is ten months where it reaches the stratosphere easily within this timeframe. Chloromethane is harmful to the environment as it mixes with various natural sinks to reach all the land, air, and water ecosystems. Chloromethane is a stable molecule but being in high concentration and the ever-changing climatic pattern has what made it a deadly one to deal with altogether. The Lewis structure is a pictorial representation showing the electrons present in the valence shell in an atom. The diagram is drawn to determine how the valence electrons of different atoms participate in the bond formation to form a molecule. If you realize, these valence electrons are always written in pair at each side of the symbol to show if all paired electrons exist or not. In addition to this, the Lewis structure also determines whether a single, double or triple bond is formed between the interacting atoms. To begin with the Lewis structure of CH3Cl, first, we need to determine the electronic configuration of each participating atom. The atomic number of Hydrogen H is 1, so its electronic configuration is 1s1.
Labview run time engine 2011
Basic skeletal of CH 2 Cl 2 is shown below. The carbon, chlorine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The CH3Cl molecule has a permanent dipole moment due to an unequal charge distribution of negative and positive charges. The C-Cl and C-H bond lengths are and pm picometer respectively. In short, now you have to find the formal charge on carbon C atom, hydrogen H atoms as well as chlorine Cl atom present in the CH3Cl molecule. Put these values for the carbon atom in the formula above. Chlorine is the second member of the halogen family. This indicates that the above lewis structure of CH3Cl is stable and there is no further change in the above structure of CH3Cl. Zero charge on the CH3Cl molecular structure. No lone pair of electrons on the carbon atom in the tetrahedral geometry of the CH3Cl molecule. The CH3Cl molecule has one central carbon, three hydrogen, and one chlorine atoms. The electronegativity value in periodic groups grows from left to right in the periodic table and drops from top to bottom. Then place no electrons as a lone pair of electrons on the carbon atom of the CH3Cl molecule. Furthermore, carbon has a four electrons limit since chlorine is the most electronegative element in the CH3Cl molecule.
Chloromethane CH 3 Cl contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom.
CH 3 Cl lewis structure There are three hydrogen atoms around center carbon atom. Chloromethane is used as an organic volatile solvent in organic reactions. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. One chlorine and three hydrogen atoms establish covalent connections with the central carbon atom as a result, leaving the carbon atom with no lone pair. Information on chloromethane CH3Cl. Carbon is group 14 element on the periodic table. To calculate the formal charge on the central carbon atom of the CH3Cl molecule by using the following formula:. The electronegativity of an atom is the strength with which it may attract bound electron pairs to its side. In this post, we discussed the method to construct the CH3Cl Lewis structure. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. The central carbon atom undergoes octet stability.

I consider, what is it � a lie.