Electron rich hydrides
Hydrides - Hydrides are the binary compound of hydrogen with other elements. Let 'X" be any element, then. Ex - MgH 2 is the hydride of Magnesium.
N H 3 is electron rich hydride. Describe electron-rich hydride. Which of the following hydrides is an electron-rich hydride? Name two electron rich hydrides. Write the names of any two electron - rich hydrides. Which element from electron rich hydride?
Electron rich hydrides
Define the following terms: a electron-deficient b electron - precise c electron-rich compound. Justify your answer with an example? Example: CH 4 , SiH 4 , etc. What do you understand by i electron-deficient, ii electron-precise, and iii electron-rich compounds of hydrogen? Provide justification with suitable examples. Byju's Answer. Open in App. Electron deficient: Electron-deficient compounds are those compounds that don't have a sufficient number of electrons to complete the octet of the central atom. These compounds contain insufficient numbers of electrons to form normal electron-pair bonds between each pair of bonded atoms. Electron deficient compounds exist in polymorphic forms to complete their electron deficiency. Example: The compounds containing less than 8 electrons in the valence shells are called electron-deficient compounds such as B 2 H 6 , AlH 3 , etc. Electron precise : Electron precise compounds of hydrogen contain sufficient valence electrons to form covalent bonds. The type of hydrides that have the exact number of electrons to form a covalent bond is called electron precise.
EMRS Accountant. BPSC Assistant. RPF SI.
.
What do you understand by i electron-deficient, ii electron-precise, and iii electron-rich compounds of hydrogen? Provide justification with suitable examples. Byju's Answer. Molecular hydrides are classified as electron deficient, electron precise and electron rich compounds. Explain each type with two examples. Open in App.
Electron rich hydrides
Hydride , in simple terms, is said to be the anion of hydrogen. It is a chemical compound where the hydrogen atoms exhibit nucleophilic, basic or reducing properties. Compounds of hydrogen with less electronegative elements are known as hydrides. So, when hydrogen reacts with any other element, the product formed is considered to be a hydride. If we closely observe the periodic table, hydride formation is not seen from VA group elements, and this condition is known as the hydride gap. Hydrogen molecule usually reacts with many elements except noble gases to form hydrides.
Free fire tips and tricks
HPCL Technician. CIL MT. Goa Police Sub Inspector. India post Postman. Odisha Police Constable. Maharashtra Zilla Parishad Stenographer. South Indian Bank Clerk. The disease caused by breathing polluted air is:. Which method can be employed to produce a high degree of homogeneity in the creation of ZnFe2O4 spinel? GATE Physics. Electron rich: The type of hydrides that have more electrons than required for bonding is called electron-rich hydrides. Vizag Steel Management Trainee. UP PGT. Central Bank of India Manager.
The combination of hydrogen with another element produces a hydride, E x H y. The formal charge or oxidation state of the hydrogen in these compounds is dependant on the relative electronegativity of the element in question.
EPFO Stenographer. MP Mahila Supervisor. Syndicate Bank PO. FCI Assistant Grade 3. NVS Electrician. Patna High Court Personal Assistant. GATE Chemistry. MP Jail Prahari. Bihar Senior Secondary Teacher. RISF Constable. Bank Note Press Dewas. Rajasthan Housing Board JE. For ex- CH 4. EMRS Accountant. These types of compounds are usually formed by groups of 15, 16, and 17 elements.

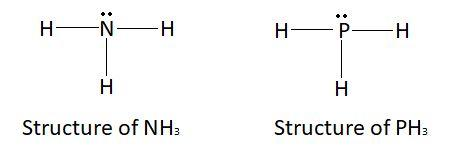
Completely I share your opinion. In it something is also idea excellent, I support.
I am sorry, that has interfered... I understand this question. Is ready to help.
In my opinion you commit an error. I can defend the position. Write to me in PM, we will discuss.