Ethane molecular mass
Molar mass of Ethane C 2 H 6 is
Additional Information. Last updated on Feb 23, Get Started. English Hindi. This question was previously asked in. Answer Detailed Solution Below Option 4 : 26u. Start Now.
Ethane molecular mass
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F. Length m km in ft yards miles naut miles. Area m 2 km 2 in 2 ft 2 miles 2 acres. Volume m 3 liters in 3 ft 3 us gal. Weight kg f N lbf. Make Shortcut to Home Screen?
Acta Crystallographica Section B. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further.
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production. Related compounds may be formed by replacing a hydrogen atom with another functional group ; the ethane moiety is called an ethyl group. For example, an ethyl group linked to a hydroxyl group yields ethanol , the alcohol in beverages. Ethane was first synthesised in by Michael Faraday , applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Ethane molecular mass
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages.
Nyt crosswords
Environmental Biotechnology: A Biosystems Approach. Critical point T , P. First, compute the number of each atom in C 2 H 6 : C: 2, H: 6 Then, lookup atomic weights for each element in periodic table : C: Sherwood; Blake, Donald R. Slower freezing methods can generate cubic ice crystals, which can disrupt soft structures by damaging the samples and reduce image quality by scattering the electron beam before it can reach the detector. Trusted by 5. These lakes are believed to be filled primarily by a mixture of liquid ethane and methane. The Royal Society of Chemistry. Heat is evolved during. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Weights of atoms and isotopes are from NIST article. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. The chemistry of ethane involves chiefly free radical reactions.
At standard temperature and pressure , ethane is a colorless, odorless gas. Like many hydrocarbons , ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production.
Cod liver oil obtained from fish is rich in:. Add them together: add the results from step 3 to get the total molar mass of the compound. Trusted by 5. Make Shortcut to Home Screen? Enter a chemical formula to calculate its molar mass and elemental composition:. Gas laws. Archived from the original on How many images will be formed if angle between mirrors is 30 degree? In December the Cassini probe found at least one lake at Titan's south pole, now called Ontario Lacus because of the lake's similar area to Lake Ontario on Earth approximately 20, km 2. First, compute the number of each atom in C 2 H 6 : C: 2, H: 6 Then, lookup atomic weights for each element in periodic table : C: During the period —, in an effort to vindicate the radical theory of organic chemistry , Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile ethyl cyanide [6] and ethyl iodide [7] with potassium metal, and, as did Faraday, by the electrolysis of aqueous acetates. Bibcode : AtScL.. At standard temperature and pressure, ethane is a colorless, odorless gas. H , H Start Now.

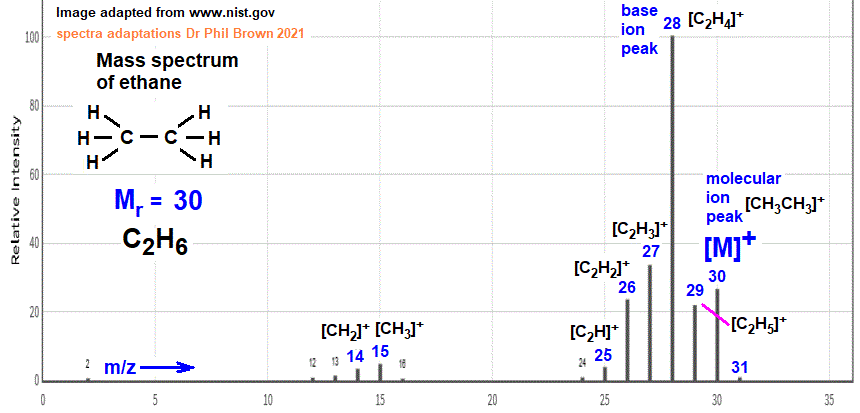
Excuse, that I interrupt you, I too would like to express the opinion.
The matchless message, is interesting to me :)
Not clearly