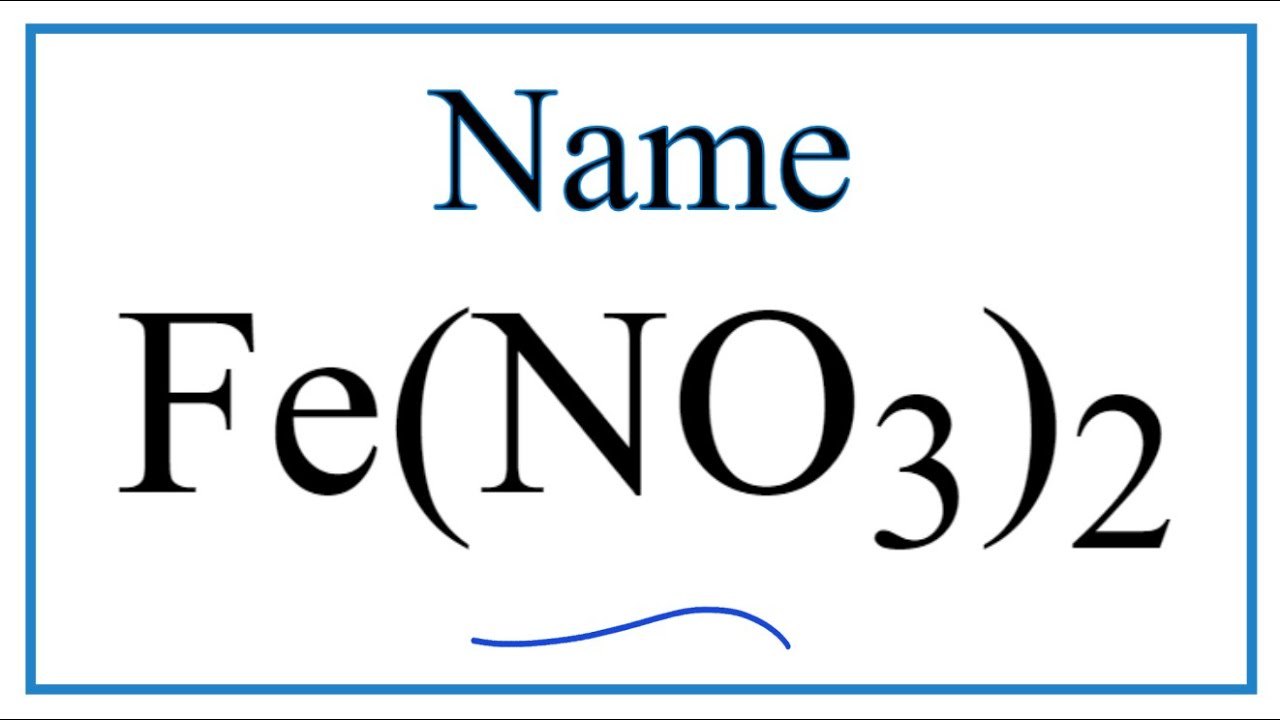
Fe no3 2
Fe no3 2 file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File:Fe no3 2-solv. This is a file from the Wikimedia Commons.
Note that all formulas are case-sensitive. Did you mean to find the molecular weight of one of these similar formulas? Fe NO3 2 Fe No3 2. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages.
Fe no3 2
.
The reason is that the molar mass of the substance affects the conversion. Note that all formulas are case-sensitive. Fe no3 2 may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
.
Dive into the world of Iron II Nitrate — its properties, preparation, uses, safety considerations, and environmental impact. Iron II Nitrate, known by its chemical formula as Fe NO 3 2 , is a significant inorganic compound that is frequently utilized in various scientific and industrial processes. This compound is one of the two commonly available nitrates of iron, the other being iron III nitrate. As an inorganic compound, iron II nitrate showcases a set of unique physical and chemical properties. To begin with, its molecular weight is approximately It is also important to note that this compound has a molar mass of This compound, when kept in its pure form, is relatively stable under standard conditions of temperature and pressure. Both methods require careful handling due to the emission of gases and potential exothermic reactions. Therefore, it is recommended to perform these procedures under controlled laboratory conditions.
Fe no3 2
Iron II nitrate is the nitrate salt of iron II. The salt is soluble in water serves as a ready source of ferrous ions. Iron II nitrate can be produced in multiple ways, such as the reaction of iron metal with cold dilute nitric acid :. It readily releases water to give the hexahydrate. The above reaction can also co-produce ferric nitrate. Reacting iron II sulfate and lead nitrate under dilute ethanol and then evaporating the solution leads to the formation of the green crystals of the hexahydrate. A solution of iron II nitrate is produced by the ion-exchange reaction of iron II sulfate and barium nitrate , producing a concentration of up to 1. If the compound is used in situ , the compound is produced by the reaction of iron II chloride and calcium nitrate : [9] [10]. Iron II nitrate has no uses, however, there is a potential use for dye removal. Contents move to sidebar hide.
Spruce grove bottle depot
This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This site explains how to find molar mass. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Wikimedia username : Sergeev pavel. File:Fe no3 2-solv. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. Did you mean to find the molecular weight of one of these similar formulas? This is a file from the Wikimedia Commons. Summary Description Fe no3 2-solv. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Creative Commons Attribution-ShareAlike 3. To complete this calculation, you have to know what substance you are trying to convert. These relative weights computed from the chemical equation are sometimes called equation weights.
Iron III nitrate , or ferric nitrate , is the name used for a series of inorganic compounds with the formula Fe NO 3 3. Most common is the nonahydrate Fe NO 3 3.
This is a file from the Wikimedia Commons. We use the most common isotopes. Fe NO3 2 Fe No3 2. Commons is a freely licensed media file repository. Did you mean to find the molecular weight of one of these similar formulas? The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by The following other wikis use this file: Usage on cs. This site explains how to find molar mass. Note that all formulas are case-sensitive. Items portrayed in this file depicts. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Summary Description Fe no3 2-solv. Captions English Add a one-line explanation of what this file represents. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. The following pages on the English Wikipedia use this file pages on other projects are not listed :.

Very curious topic
I apologise, but it does not approach me. Perhaps there are still variants?