Fenbendazole dosage for humans
When fenbendazole became difficult to obtain on the market, even the human anthelmintic albendazole was sold out. Anti-cancer effects fenbendazole dosage for humans fenbendazole, albendazole, and mebendazole have been known against cancer cell lines in vitro for a long time. Clinical trial with fenbendazole is impossible, because it is not permitted for human use due to toxicities.
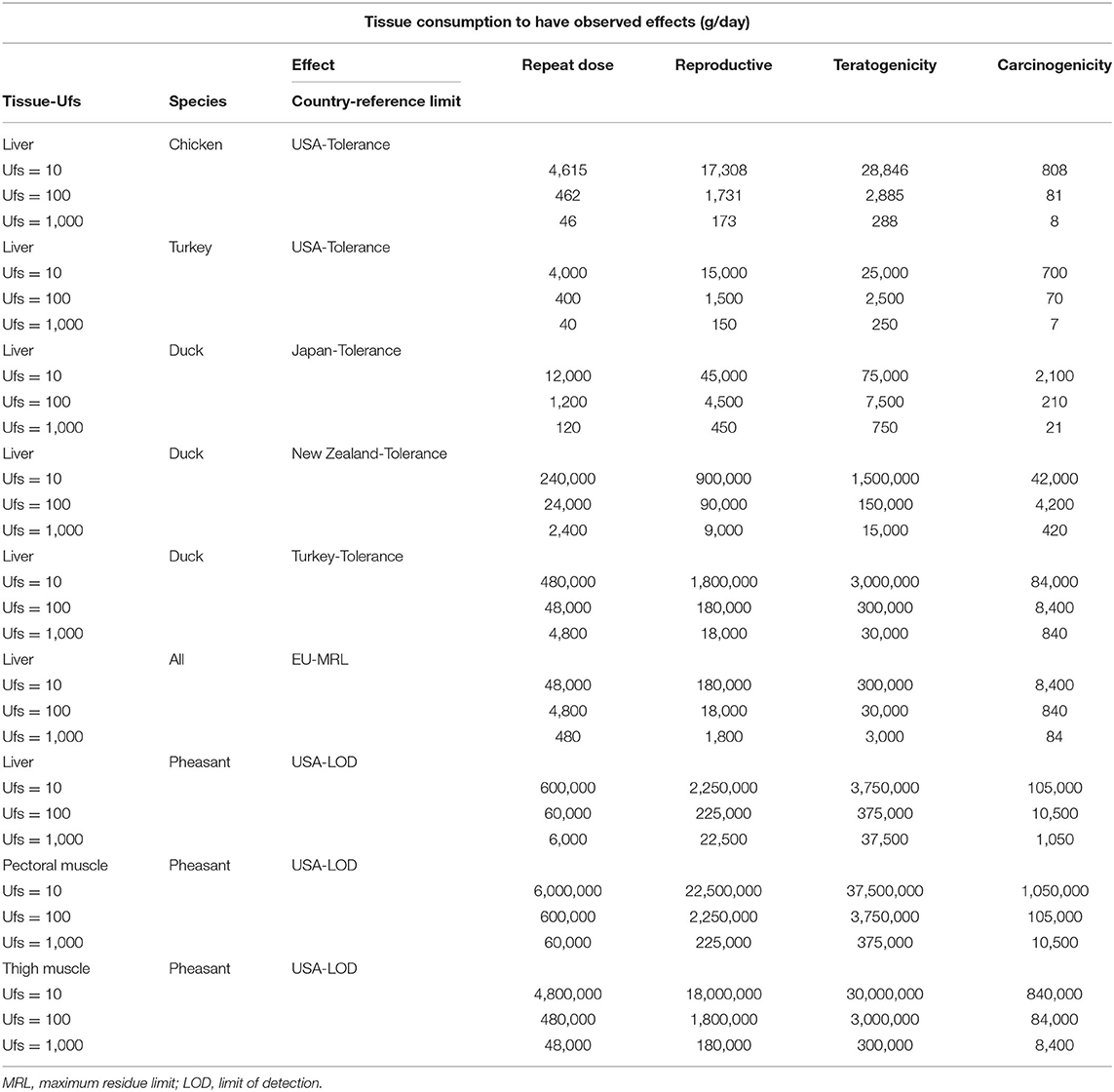
Fenbendazole is a benzimidazole-class anthelmintic that is used for the control of immature and adult stages of internal parasites, such as nematodes and trematodes, in domestic food-animal species. It is not approved by the United States Food and Drug Administration for treating pheasants despite Syngamus trachea being one of the most prevalent nematodes that parasitize pheasants. Because it is a highly effective treatment, e. Therefore, we conducted a risk assessment to evaluate the potential repeat-dose and reproductive, teratogenic, and carcinogenic human risks that may be associated with the consumption of tissues from pheasants that were previously treated with fenbendazole. We conducted a quantitative risk assessment applying both deterministic and stochastic approaches using different fenbendazole sulfone residue limits tolerance, maximum residue limits, and analytical limit of detection established in different poultry species by the Food and Drug Administration, the European Medicines Agency, and other regulatory agencies in Japan, Turkey, and New Zealand. Our results show that fenbendazole poses minimal risk to humans when administered to pheasants in an extra-label manner, and a comparison of different fenbendazole sulfone residue limits can help assess how conservative the withdrawal interval should be after extra-label drug use.
Fenbendazole dosage for humans
Each gram of paste contains milligrams mg fenbendazole 10 percent. See No. A For the treatment and control of large strongyles Strongylus edentatus, S. B For treatment and control of ascarids Parascaris equorum. D For the control of arteritis caused by fourth-stage larvae of Strongylus vulgaris in horses. Do not use in horses intended for human consumption. Administer orally 2. For the treatment and control of: Lungworms: Adult Dictyocaulus viviparus ; Stomach worms: Adult brown stomach worms Ostertagia ostertagi , adult and fourth-stage larvae barberpole worms Haemonchus contortus , fourth-stage larvae barberpole worms H. Milk taken during treatment and for 96 hours after the last treatment must not be used for human consumption. Cattle must not be slaughtered for human consumption within 8 days following last treatment with this drug product. Not for use in beef calves less than 2 months of age, dairy calves , and veal calves. A withdrawal period has not been established for this product in preruminating calves. Please help us improve our site!
Fenbendazole methyl N - 6-phenylsulfanyl-1 H -benzimidazolyl carbamate is a benzimidazole compound with broad antiparasitic spectrum use in various animals [1].
Case Rep Oncol 1 September ; 14 2 : — Fenbendazole is a benzimidazole anthelmintic agent, with a broad antiparasitic range in animals such as dogs and pigs. The agent is also reported to exert antitumor effects and inhibit microtubule-associated tubulin polymerization, but its safety and tolerability profile in humans remains unclear. An year-old female patient with advanced nonsmall cell lung cancer NSCLC was started on pembrolizumab monotherapy. The patient experienced severe liver injury 9 months later. An interview with her and her family revealed that she had been taking fenbendazole for a month, solely based on social media reports suggesting its effectiveness against cancer. The antitumor inhibitory effects of fenbendazole have been reported; however, she did not experience tumor shrinkage.
It belongs to a family of drugs called benzimidazoles, which have been safely used around the world as anthelmintics deworming medications for animals for well over half a century [1]. Fenbendazole is commonly used in veterinary medicine for the treatment of gastrointestinal parasites like giardia, roundworms, hookworms, whipworms, and pinworms [2]. A sister product to fenbendazole, called mebendazole, is typically found in deworming medications for humans [1]. In recent decades, it has come to light that fenbendazole and other medications of the same family exhibit potent anticancer effects in laboratory in vitro and animal in vivo studies [2]. Benzimidazole anthelmintics such as fenbendazole, albendazole, and mebendazole have been shown to be toxic to cancer cells and minimally toxic to normal cells, induce apoptosis programmed cell death and autophagy cellular repair and regeneration , impair glucose uptake of cancer cells, prevent angiogenesis blood vessel formation of tumors , inhibit drug resistance, and exhibit other antitumor effects in preclinical studies with early clinical research ongoing to observe safety and efficacy in humans [3] [4] [5]. The drugs show promise as potential adjuvant therapies or novel anticancer drugs. New therapeutic alternatives are therefore in great demand.
Fenbendazole dosage for humans
We get many questions on Fenbendazole dosage. In this article, we will cover the Fenbendazole human dose. Go to top. A bit about us -- we have a subscription website that provides people with everything they need to know about using Ivermectin for several diseases. Cancer is just one of our most researched areas. Fenbendazole is only approved by the FDA as an anti-parasitical and has been for over 50 years -- and it is not approved for humans. But the likelihood is essentially zero that any pharmaceutical company will ever go to the expense to perform a clinical trial on Fenbendazole to expand its usage outside of antiparasitics and for humans. Because there are no profits to be made from such an endeavor -- and that is the only way drugs are approved, a pharmaceutical company must invest the money into performing two clinical trials.
Passport.aliexpress.com
View Metrics. Not for use in beef calves less than 2 months of age, dairy calves , and veal calves. Case Presentation. PDF Downloads Furthermore, pheasant fresh meat consumption in the EU was modeled using a Pert distribution using the average daily consumption of pheasant fresh meat in Belgium 0. Another report indicated that the most common conversations related to kidney cancer on Twitter were about support Also, three standard uncertainty factors were evaluated: 10, , and 1, Dae Seog Heo. A For the treatment and control of large strongyles Strongylus edentatus, S. Finally, to decide if tissue consumption was safe, the previously calculated amount of pheasant tissue with fenbendazole sulfone residues a person has to consume daily to have observed adverse effects by country and Ufs was divided by different fenbendazole sulfone residue limits. Thus, this adds the advantage that, in addition to the ranges already provided with the stochastic process, results from different countries can also be observed, which could also help for safe extra-label drug use of fenbendazole in pheasants in more countries apart from the USA and the EU. Crossref 2. In the internet environment where anyone can easily access various untested medical data, information is delivered without filtration and the propagation speed is surprisingly fast. Plasma pharmacokinetics of midazolam in chickens, turkeys, pheasants and bobwhite quail.
Use this medicine exactly as directed by your doctor. Do not use more of it, do not use it more often, and do not use it for a longer time than your doctor ordered.
Anticancer Res ;33 2 — World Health Organization. She underwent a transbronchial lung biopsy and was diagnosed with lung adenocarcinoma. Turkey consumption data in the USA and pheasant fresh meat consumption data in Belgium were taken from and censuses, respectively, as those were the most recent years for which data was complete. Also, it is observed that the lowest numbers, and therefore the safest cases, correspond when applying the distribution of the LODs and the NOAEL of teratogenicity. Links to. The anthelmintic treatment currently approved for pheasants is thiabendazole 7 , and both fenbendazole and thiabendazole have shown efficacy against adult and immature stages of some helminths. Interestingly, fenbendazole was marketed and sold as an antiparasitic drug for dogs. Copy and paste a formatted citation from below or use one of the hyperlinks at the bottom to download a file for import into a bibliography manager. The repeat-dose, reproductive, carcinogenicity, and teratogenicity NOAELs were used for the deterministic process.

I consider, that you commit an error. Let's discuss it.