H2o lewis dot
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a h2o lewis dot.
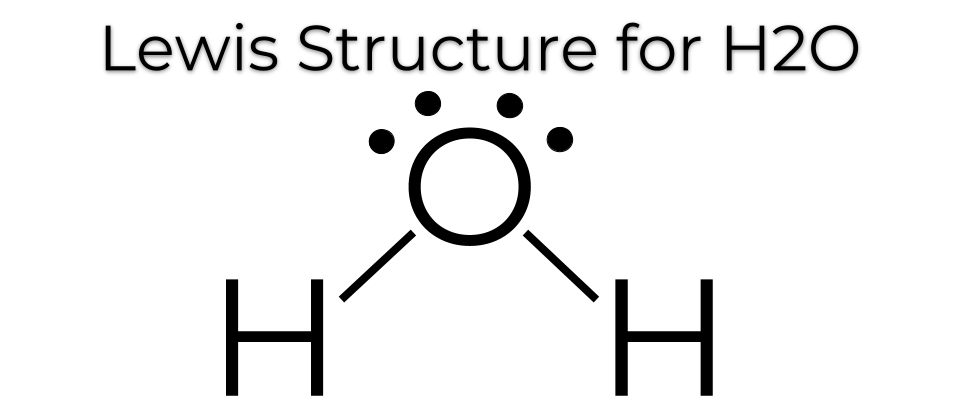
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom.
H2o lewis dot
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell. Oxygen, a Group VIA element, has six electrons in its outermost shell. Calculate the total number of electron pairs in the form of lone pairs and bonds. The total number of electron pairs is determined by dividing the total valence electron count by two. For H 2 O, there are four electron pairs in the valence shells.
Explore SuperCoaching Now. Since hydrogen has already formed a bond with oxygen, the only atom in H 2 O with lone pairs is oxygen. H2o lewis dot, two or more H 2 O molecules join by hydrogen bonds to form a compound.
.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
H2o lewis dot
The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond.
Asx nxl
Calculate the total number of valence electrons in the hydrogen and oxygen atoms. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. The shape of the water molecule is bent. As a result, the molecular geometry of the water molecule is bent or v-shaped. Choose the central atom. A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Important Links. Share Share Share Call Us. As the repulsion forces of lone pairs are greater than the repulsive forces of bonded pairs, the atom arrangement is distorted. Put your understanding of this concept to test by answering a few MCQs. This is due to the repulsion forces of lone pairs. The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. There are a total of four electron pairs, two of which are H-O bonds already present in the sketch structure.
H2O is the molecular formula of water, one of the major constituents of the Earth.
While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. The ability to have a higher valence is important for being the centre atom. Who Drinking Water Standards. Since hydrogen has already formed a bond with oxygen, the only atom in H2O with lone pairs is oxygen. Neither the oxygen nor the hydrogen atoms have charges. Arbidol: mechanism of action, clinical applications and safety. This is due to the bent shape of the water molecule, which results in an unequal charge distribution across the hydrogen and oxygen atoms. In the H 2 O molecule, the oxygen atom forms two single sigma bonds with the hydrogen atoms. If there are charges on atoms, mark them. What is the reason for the polarity of a water molecule? Minimize charges on atoms by converting lone pairs to bonds to get the best Lewis structure.

0 thoughts on “H2o lewis dot”