H2o molar mass
Molar mass of H 2 O Water is Then, lookup atomic weights for each element in periodic table : H: 1.
Water is an inorganic compound with the chemical formula H 2 O. It is a transparent, tasteless, odorless, [c] and nearly colorless chemical substance , and it is the main constituent of Earth 's hydrosphere and the fluids of all known living organisms in which it acts as a solvent [19]. It is vital for all known forms of life , despite not providing food energy or organic micronutrients. Its chemical formula, H 2 O , indicates that each of its molecules contains one oxygen and two hydrogen atoms , connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of Because Earth's environment is relatively close to water's triple point , water exists on Earth as a solid , a liquid , and a gas. Clouds consist of suspended droplets of water and ice , its solid state.
H2o molar mass
Last updated on Mar 15, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt. Police Exams. Insurance Exams. Nursing Exams.
Archived from the original on 5 October
Or you can choose by one of the next two option-lists, which contains a series of common organic compounds including their chemical formula and all the elements. The molecular mass calculator will recognize the entered formula's, which are included in the list of organic compounds. The calculator handles at most two different bracket levels. Make sure you enter the molecule of crystallization at last e. Elements of the periodic table.
Molar mass of H 2 O Water is Then, lookup atomic weights for each element in periodic table : H: 1. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in H 2 O: H: 2, O: 1 Then, lookup atomic weights for each element in periodic table : H: 1. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
H2o molar mass
This compound is also known as Water or Dihydrogen Monoxide. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages.
Walmart neighborhood market kernersville nc
Archived PDF from the original on 29 May Freshwater is a renewable resource, recirculated by the natural hydrologic cycle , but pressures over access to it result from the naturally uneven distribution in space and time, growing economic demands by agriculture and industry, and rising populations. Retrieved 24 September European Journal of Clinical Nutrition. Life's matrix : a biography of water. He was born in England on December 25, This section needs additional citations for verification. Containerization revolutionized maritime transport starting in the s. When the composition is expressed as a molality , the proportionality constant is known as the cryoscopic constant K f and is characteristic for each solvent. Then, lookup atomic weights for each element in periodic table : Li: 6. BMTC Conductor. Reverse osmosis RO desalination plant in Barcelona , Spain.
I want to make a solution that contains 1. You do not have a balance calibrated in moles, but you do have one calibrated in grams. If you know the relationship between moles and the number of grams in a mole, you can use your balance to measure out the needed amount of material.
In Shinto, water is used in almost all rituals to cleanse a person or an area e. It becomes positively charged. Molar masses typically vary between:. Without water, these particular metabolic processes could not exist. Archived PDF from the original on 15 November Main articles: Relative atomic mass and Standard atomic weight. Yellowstone National Park: U. Mole concepts. Temperature Unit Conversion. Common impurities include metal salts and oxides, including copper, iron, calcium and lead, [] [ full citation needed ] and harmful bacteria, such as Vibrio. Archived from the original on 22 February Chemical formula:. Gram atomic mass is another term for the mass, in grams, of one mole of atoms of that element. The isotopic distributions of the different elements in a sample are not necessarily independent of one another: for example, a sample which has been distilled will be enriched in the lighter isotopes of all the elements present.

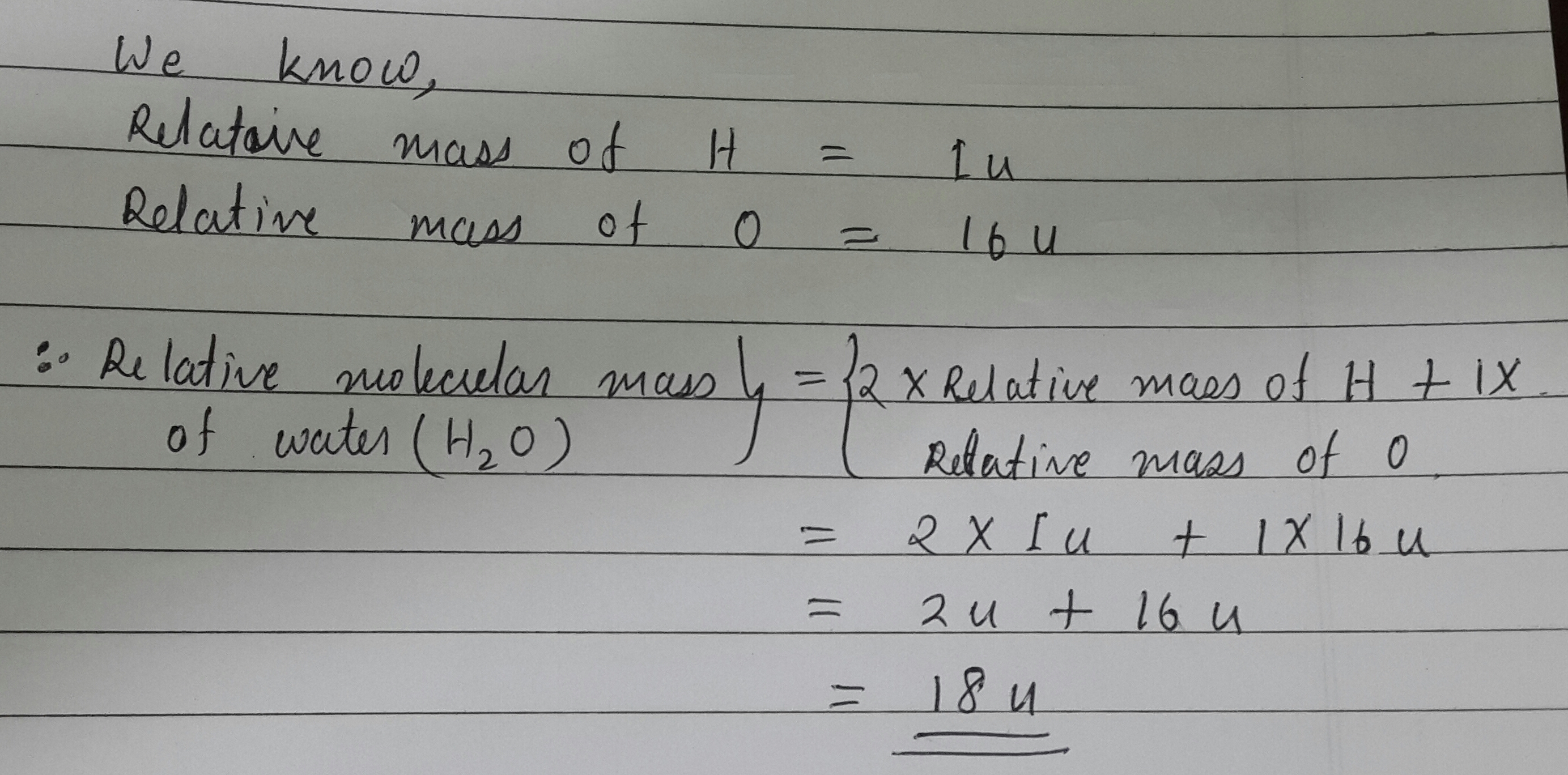
0 thoughts on “H2o molar mass”