Hcl + koh reaction
Direct link to this balanced equation:. A chemical equation represents a chemical reaction.
Submitted by Joseph M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. If hydrochloric acid HCl reacts with the base lithium hydroxide LiOH , what are the products of the reaction?
Hcl + koh reaction
.
Write a chemical equation for the reaction. Ask unlimited questions and get video answers from our expert STEM educators.
.
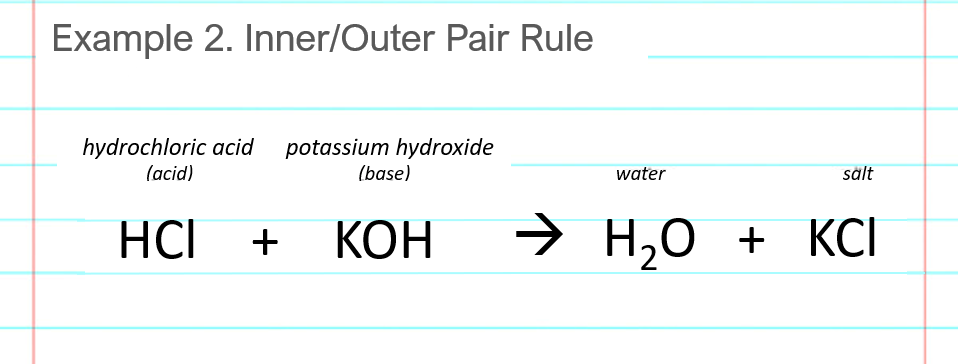
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base. These original definitions were proposed by Arrhenius the same person who proposed ion dissociation in , so they are referred to as the Arrhenius definition of an acid and a base, respectively. Do we really have bare protons moving about in aqueous solution? The reaction of an acid and a base is called a neutralization reaction. In fact, the general reaction between an acid and a base is.
Hcl + koh reaction
Acid—base reactions are essential in both biochemistry and industrial chemistry. Moreover, many of the substances we encounter in our homes, the supermarket, and the pharmacy are acids or bases. For example, aspirin is an acid acetylsalicylic acid , and antacids are bases.
El manaba fotos
Don't have an account? Cancel Send Feedback. Chemistry tools. Gas laws. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. O is balanced: 1 atom in reagents and 1 atom in products. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. H is balanced: 2 atoms in reagents and 2 atoms in products. Chemical forum. Process: split the reaction into two half-reactions, balance the atoms and charges in each half-reaction, and then combine the half-reactions, ensuring that electrons are balanced. Ask your parent or guardian for help. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Previous Next: balancing chemical equations.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction.
It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Gas laws. A chemical equation represents a chemical reaction. Computer Science Chemistry Physics. Unit converters. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Direct link to this balanced equation:. In many cases a complete equation will be suggested. Sign Up. The equation is balanced. This is the most straightforward method. This method uses algebraic equations to find the correct coefficients. Login Sign up. Balancing with inspection or trial and error method This is the most straightforward method. The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character.

It is a pity, that now I can not express - it is very occupied. I will be released - I will necessarily express the opinion on this question.
Your idea simply excellent