Heating of ferrous sulphate crystal
Thus, the crystals of ferrous sulphate on heating give ferric oxide, sulphur dioxide and sulphur trioxide. Byju's Answer. Open in App.
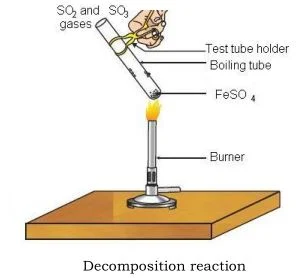
Viva Voce. Decomposition Reaction. Materials required: Procedure: Real Lab Procedure: Take about 2g of ferrous sulphate crystals in a dry boiling tube and note the colour of the crystals. Hold the boiling tube with a test tube holder and heat the boiling tube over the flame of a burner. Smell the gas being emitted. Observe the colour of the crystals after heating.
Heating of ferrous sulphate crystal
Find the answer to this question and access a vast question bank that is customized for the student. Explanation: —. FeSO 4. When ferrous sulphate is heated, it loses its water molecules and creates an anhydrous ferrous sulphate molecule, as well as changing the color of the ferrous sulphate molecule from green to white. Complete the following steps in order:. A decomposition reaction occurs when a chemical compound is broken down into two or more other compounds. The double decomposition reaction occurs when reactants exchange positive and negative ions, resulting in the synthesis of new molecules. Thermal, electrolytic, and pyrolytic decomposition reactions are the three types of decomposition reactions. When ferrous sulphate is heated, it loses its water molecules and forms anhydrous ferrous sulphate, which changes the color of the crystal from light green to white. When ferrous sulphate is heated further, it breaks down into ferric oxide, Sulphur trioxide, and Sulphur dioxide. The following are the reactions that occurred:.
Note: Thermal breakdown reaction is the process by which ferrous sulphate decomposes.
.
Thus, the crystals of ferrous sulphate on heating give ferric oxide, sulphur dioxide and sulphur trioxide. Byju's Answer. Open in App. The reaction of ferrous sulphate upon heating: The water of crystallisation is present in ferrous sulphate crystals FeSO 4. Ferrous sulphate crystals lose water when heated.
Heating of ferrous sulphate crystal
Effect of concentration and temperature variation on the rate of chemical reaction. Enthalpy Change for the Interaction between Acetone and Chloroform. Preparation of mL of 0. Study the Shift in Equilibrium between Ferric ions and Thiocyanate ions. Identify Bleaching Powder among the given Samples of Chemicals. Crystals of copper sulphate contain water of crystallization. Esterification Reaction between Alcohol and Carboxylic Acid. Measure the change in Temperature during Chemical Reactions. To show that Gases are readily Compressible and Liquids are not.
Waynesboro va population 2023
Open in App. Write chemical equation of the reaction. On further heating, anhydrous ferrous sulphate decomposes to form ferric oxide Fe 2 O 3 , sulphur dioxide SO 2 and sulphur trioxide SO 3. The gases SO 2 and SO 3 are very harmful, so do not take a deep breath when smelling the odour of the gases. Trending Questions. The following are the reactions that occurred:. Hold the boiling tube with a test tube holder and heat the boiling tube over the flame of a burner. Adjust the resistance of the rheostat using the slider. To turn on the burner, click on the knob of the burner. When ferrous sulphate is heated, it loses its water molecules and creates an anhydrous ferrous sulphate molecule, as well as changing the color of the ferrous sulphate molecule from green to white.
The positions of the hydrogen atoms were located based on DFT calculations. IR and Raman spectra are presented and discussed according to this new structure model.
Standard XII Chemistry. So their colour changes from light green to white. Because ferrous sulphate decomposes into many products in this reaction, it is classified as a decomposition reaction. On further heating, anhydrous ferrous sulphate decomposes to form ferric oxide Fe 2 O 3 , sulphur dioxide SO 2 and sulphur trioxide SO 3. Note: Thermal breakdown reaction is the process by which ferrous sulphate decomposes. When ferrous sulphate is heated, it loses its water molecules and forms anhydrous ferrous sulphate, which changes the color of the crystal from light green to white. To heat the contents of the test tube, drag the test tube over the burner. Observe the colour of the crystals after heating. Precautions: Do not point the mouth of the boiling tube at your neighbours or yourself. The reaction of ferrous sulphate upon heating: The water of crystallisation is present in ferrous sulphate crystals FeSO 4. Find the answer to this question and access a vast question bank that is customized for the student. The following are the reactions that occurred:. FeSO 4. Oxides of Nitrogen.

0 thoughts on “Heating of ferrous sulphate crystal”