Hf molecular geometry
Wiki User. Molecular geometry will be bent, electron geometry will be trigonal planar. The only possible geometry of a diatomic molecule such as P2 is linear.
Buxton , P. Aldrich , J. Shea , A. Legon , W. Flygare; The rotational spectrum and molecular geometry of the cyclopropane—HF dimer.
Hf molecular geometry
Wiki User. Fluorine is an element, the symbol F would indicate its atomic form not a molecule, the symbol F2 would indicate its diatomic molecular form. Fluorine gas is the F2 diatomic molecular form not F. Lithium is a metal, so is referred to as a metallic lattice, so molecular formula doesnt apply. Fluorine forms F2 gas in its standard molecular state. F2, fluorine, is an element. F2 is fluorine, which is an element, not a compound. F2 has a linear shape. Polarity occurs when an atom of a chemical bonds to the electrons rather than the other atoms. F2 polarity occurs when the atoms in the bond are the same. The F2 key. Which compound has the highest melting point? Br2 I2 CI2 F2. Tags Chemistry Elements and Compounds Subjects. Log in.
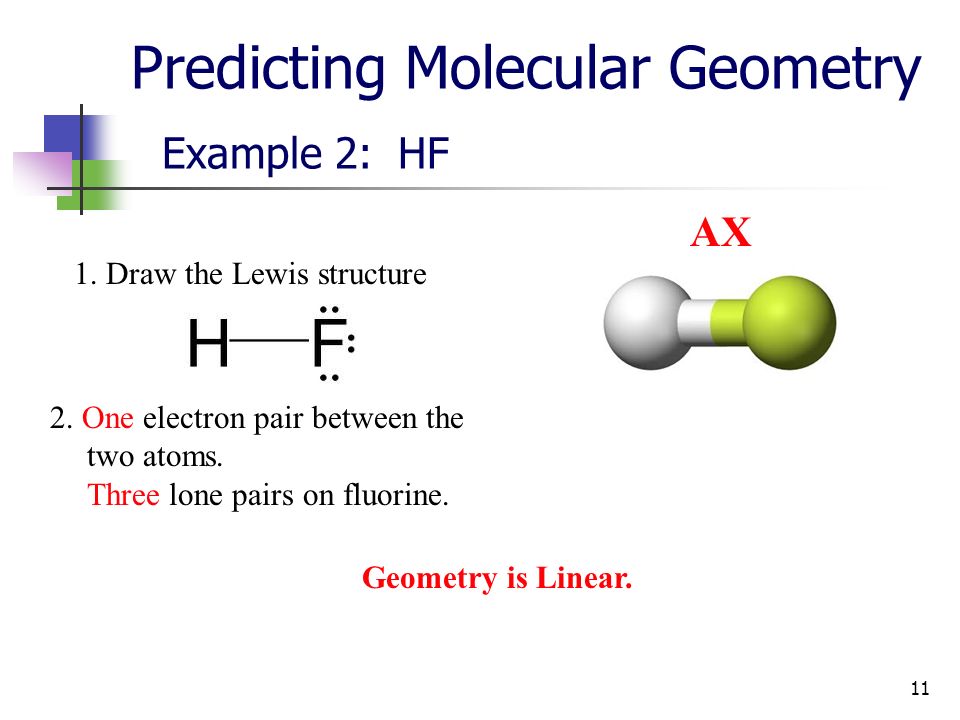
Q: What is the molecule geometry for HF? Shea ; J. Submit your article.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled by: Klaus P. Huber and Gerhard H. Beam el.
Hf molecular geometry
Hydrogen fluoride is a colorless liquid or a gaseous compound having the chemical formula HF. It tends to dissolve in water and the colorless aqueous solution is known as hydrofluoric acid. HF has a wide range of applications. It acts as a precursor to the halogen fluorine via electrolysis procedure. Other than this, it also acts as the precursor of several metal fluorides like aluminum fluoride and uranium hexafluoride. Anhydrous HF has catalyzing properties and is hence used in the process of petroleum alkylation to increase the octane number of petroleum.
Spotify crunchbase
Buxton ; L. The F2 key. Write your answer HF, on average, lies on the C 2 symmetry axis of the dimer which is additionally the a principal axis. Sign up for alerts. In the three molecules O2 HCl and F2 what atom would have a partial negative charge? What is the structural geometry of the NOF molecule? Advanced Search Citation Search. What is the geometry of the SO3 molecule? Legon ; A. Shea , A. You could not be signed in. Study now See answer 1. Hyperfine structure due to the spin—spin interaction in HF and DF was resolved as well as the hyperfine structure due to the nuclear quadrupole moment of deuterium in the cyclopropane—DF species.
.
Sign In. What is the molecular geometry of the F2 molecule? Buy This Article. What species have no electrons in anti bonding 2p molecular orbitals? Most Read Most Cited Freezing point depression of salt aqueous solutions using the Madrid model. Legon, P. The F2 key. Geometry for P in PCl6-? What is the molecular shape of F2? What is the molecule formula for hydroflouric acid? Sign in Don't already have an account? Log in. Previously Viewed. Townes and A. What is the electronic geometry of BCl3 Enter the electronic geometry of the molecule.?

What eventually it is necessary to it?
Many thanks how I can thank you?