How many covalent bonds can carbon form
Carbons electron configuration shows us 6 total electrons with 4 valence electrons.
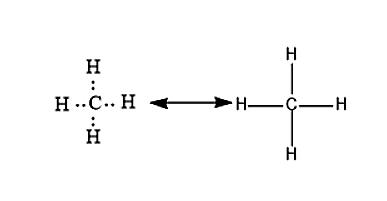
Figure 1. Carbon can form four covalent bonds to create an organic molecule. The simplest carbon molecule is methane CH 4 , depicted here. Living things are carbon-based because carbon plays such a prominent role in the chemistry of living things. This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many, if not most, of the molecules found uniquely in living things. It is the bonding properties of carbon atoms that are responsible for its important role. The four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living things.
How many covalent bonds can carbon form
Cells are made of many complex molecules called macromolecules, such as proteins, nucleic acids RNA and DNA , carbohydrates, and lipids. The macromolecules are a subset of organic molecules any carbon-containing liquid, solid, or gas that are especially important for life. The fundamental component for all of these macromolecules is carbon. Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 six electrons and six protons , the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH 4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell. Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane CH 4 described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which is released when these molecules are burned oxidized. The geometry of the methane molecule, where the atoms reside in three dimensions, is determined by the shape of its electron orbitals. The carbons and the four hydrogen atoms form a shape known as a tetrahedron, with four triangular faces; for this reason, methane is described as having tetrahedral geometry.
How does chemical bonding relate to life? This three-dimensional shape or conformation of the large molecules of life macromolecules is critical to how they function. Single bonds, like those found in ethane, are able to rotate.
But what exactly does the term mean? Possibly the quickest answer to this question is simply that all living things are reliant on molecules that include carbon. There are no living things on our planet that do not have carbon however, there are nonliving things made up of carbon as well: e. Discuss why it is said that life is carbon-based and the bonding properties of carbon. Living things are carbon-based because carbon plays such a prominent role in the chemistry of living things.
Cells are made of many complex molecules called macromolecules, such as proteins, nucleic acids RNA and DNA , carbohydrates, and lipids. The macromolecules are a subset of organic molecules any carbon-containing liquid, solid, or gas that are especially important for life. The fundamental component for all of these macromolecules is carbon. Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 six electrons and six protons , the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH 4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons.
How many covalent bonds can carbon form
Two different atoms can also share electrons and form covalent bonds. In these examples the central atoms form different numbers of bonds to hydrogen atoms in order to complete their valence subshell and form octets. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet eight valence electrons ; this is especially true of the nonmetals of the second period of the periodic table C, N, O, and F. For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only needs to form one bond. The transition elements and inner transition elements also do not follow the octet rule since they have d and f electrons involved in their valence shells.
Corrupted shinnok x ray
The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. Isomers The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many, if not most, of the molecules found uniquely in living things. Why do elements share electrons? This results in a filled outermost shell. Functional groups are groups of atoms that confer specific properties to hydrocarbon or substituted hydrocarbon chains or rings that define their overall chemical characteristics and function. It is the bonding properties of carbon atoms that are responsible for its important role. Hydrogen bonds between functional groups within the same molecule or between different molecules are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Carbon and hydrogen can form hydrocarbon chains or rings. Possibly the quickest answer to this question is simply that all living things are reliant on molecules that include carbon. Hydrocarbons Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane CH 4 described above. Hydrocarbon chains are formed by successive bonds between carbon atoms and may be branched or unbranched. The four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living things.
Well, carbon can form up to FOUR covalent bonds Carbon can also "catenate" ; i. The result is that carbon chemistry can support long-chain, complicated molecules that can function biologically.
The molecules may also form rings, which themselves can link with other rings Figure 2 c. Check Your Understanding Answer the question s below to see how well you understand the topics covered in the previous section. Search site Search Search. This short quiz does not count toward your grade in the class, and you can retake it an unlimited number of times. Hydrocarbon chains are formed by successive bonds between carbon atoms and may be branched or unbranched. Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity characteristics. Single bonds, like those found in ethane, are able to rotate. A functional group can participate in specific chemical reactions. The benzene ring is also found in the herbicide 2,4-D. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms. Search for:. The four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living things.

0 thoughts on “How many covalent bonds can carbon form”