How many electrons are in chlorine
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Chlorine Facts and Science. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Chlorine has an atomic number of 17 and an atomic mass of
Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number. In every stable atom the number of electrons is equal to the number of protons. Therefore, the number of electrons is equal to the number of protons in an chlorine atom.
How many electrons are in chlorine
Examples of Compounds with Chlorine Sodium Chloride This is sodium chloride , also known as table salt. Most people scientist know that the formula for salt is NaCl. One sodium Na atom gives it's electron to one chlorine Cl atom. Chlorine then has the eight electrons in its outer shell to make it "happy". Sodium is "happy" because it has now given up its one extra electron. Chem4Kids Sections. See the full list of chemistry topics at the site map! Current Page: Chem4Kids. They are paid advertisements and neither partners nor recommended web sites. Also, we do not collect or ask for personally identifiable information on any of our sites. Sodium Chloride This is sodium chloride , also known as table salt. Aluminum Trichloride Chlorine Cl can also bond with aluminum Al. Aluminum has three extra electrons and will easily let the chlorine atoms use them.
Chlorine Facts and Science.
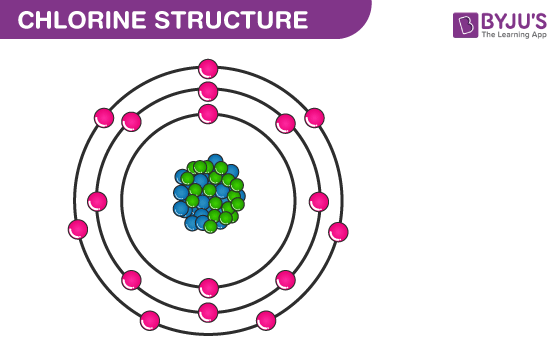
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital.
In fact, the table mentioned below is the perfect information box Which gives you every single detail about the Chlorine element in Periodic table. See how this Interactive Periodic Table helps you. Chlorine element is in group 17 and period 3 of the Periodic table. Chlorine is the p-block element and it belongs to halogens group. Click on above elements in Periodic table to see their information or Visit Interactive Periodic Table which shows names, symbol, atomic mass, electron configuration, electrons arrangement, etc.
How many electrons are in chlorine
Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate. From the given table, for energy level 1, there's only 1 sublevel, which is called 1s. In it, there's only 1 orbital. Since 1 orbital can hold at most 2 electrons, therefore 1s can hold max 2 electrons. Electrons are filled up from the lowest energy level to the highest.
Via inn shinjuku
Chlorine has 7 valence electrons. Related questions How do valence electrons affect chemical bonding? Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Shivangi Mangotra. Here is a video which gives a quick discussion of how to determine how many valence electrons atoms of different elements have. The electronic configuration of chlorine atom is:- E. Disinfection—Bleach solutions are used extensively in restaurants, schools, hospitals, homes, and other settings to disinfect surfaces, destroying pathogens, including norovirus, hepatitis A, Ebola, influenza, and many more. Stable means that atom has not formed a ion yet. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. Elemental chlorine gas Cl 2 is manufactured using the chlor-alkali process, which uses electrolysis to transform highly concentrated salt water brine into chlorine, sodium hydroxide, and hydrogen. Video: Chlorine Electron Configuration Notation. The name trichloride means three chlorine atoms are involved. The density of chlorine is
Chlorine is a yellow-green gas at room temperature.
You can reuse this answer Creative Commons License. The density of chlorine is This makes it easier to understand and predict how atoms will interact to form chemical bonds. This means, that the number of electrons present in an chlorine atom is How many valence electrons are in carbon? How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. Therefore, there are 7 valence electrons in an chlorine atom. Video from: Noel Pauller. Here is a video which gives a quick discussion of how to determine how many valence electrons atoms of different elements have. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Elemental chlorine gas Cl 2 is manufactured using the chlor-alkali process, which uses electrolysis to transform highly concentrated salt water brine into chlorine, sodium hydroxide, and hydrogen.

I am sorry, it at all does not approach me.
I congratulate, this brilliant idea is necessary just by the way
In my opinion you are mistaken. I suggest it to discuss. Write to me in PM.