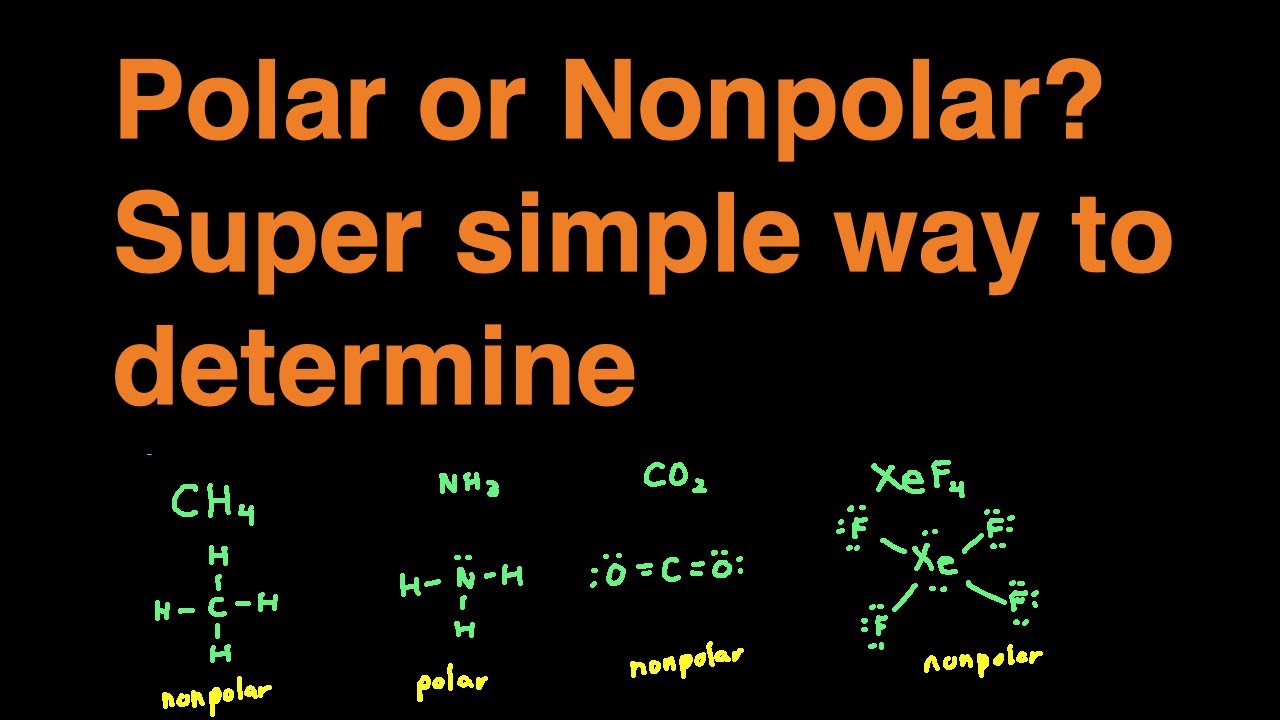
How to tell if something is polar or nonpolar
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. As their applications are on a very large scale, it has become necessary to acquire a more detailed understanding of their environmental fate. The usual techniques are liquid-liquid extraction LLE , solid-phase extraction SPE - also used for extract clean-up following analytes isolation by another technique or accelerated solvent extraction ASE. Nowadays, high-performance liquid chromatography HPLC is usually coupled with a universal mass spectrometry detector MS or tandem mass spectrometry detector MS-MS , what allows for detection, identification and quantification the various compounds in a particular group of surfactants in suitably prepared solvent extracts. Log in to send the author a request to share.
How to tell if something is polar or nonpolar
Crash Course Chemistry Sezon 1. In 46 episodes Hank Green will teach you chemistry! This course is mostly based on the AP Chemistry curriculum. Filmy dokumentalne. Ten film jest obecnie niedostępny do obejrzenia w Twojej lokalizacji. Share Android. Odcinki Szczegóły. Odcinki Sortuj Numer odcinka Najnowsze odcinki Dostępne do obejrzenia. Hank does his best to convince us that chemistry is not torture but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton neutron and electron - come together in trillions of combinations to form To wideo jest obecnie niedostępne. A unit is a frequently arbitrary designation we have given to something to convey a definite magnitude of a physical quantity and every quantity can be expressed in terms of the seven base units that are contained in the international system of units. Today's Crash Course Chemistry takes a historical perspective on the creation of science which didn't really exist until a super-smart super-wealthy Frenchman put the puzzle pieces together - Hank tells the story of how we went from alchemists to chemists.
The molecules of carbon dioxide are mostly adsorbed near the organic linkers, avoiding the metal clusters containing -OH groups which are the preferential adsorption sites for water molecules
.
Polarity is one of those properties that impact all the other physical properties that include its boiling point, melting point, and even solubility. The property is essential to know all the other chemical properties and their uses and limitations of using. But before diving into the process of how to tell if a molecule is polar or not, let us quickly go through the definition of polarity for your better understanding. Polarity: It is a separation of electric charges in the molecule due to the electric dipole moment. Such separation of charges leads to the positively charged end of a molecule and the negatively charged end. These two polar ends act as electric poles that differ in electric charges. This property of the molecule having different electric charges is termed polarity. Depending upon the electric charges of the molecule, it is classified as polar or nonpolar. To know how they exactly differ from each other, check out these differences between polar and nonpolar molecules. Polar molecules: Water, alcohol, Sulphur dioxide, ammonia , ethanol, hydrogen sulfide, bent molecules those with a significant bond angle in general.
How to tell if something is polar or nonpolar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity? Scientists have devised a scale called electronegativity , a scale for judging how much atoms of any element attract electrons. Electronegativity is a unitless number; the higher the number, the more an atom attracts electrons. Electronegativities are used to determine the polarity of covalent bonds.
Archive of ourown
The full set of charges and parameters for the L-J potentials for guests and host is shown in Tables S3 and S4 of the Supporting information. Supplementary Data 4. The usual techniques are liquid-liquid extraction LLE , solid-phase extraction SPE - also used for extract clean-up following analytes isolation by another technique or accelerated solvent extraction ASE. UiO samples were deposited as thin layers on a silicon wafer by evaporating a few drops of the MOF suspension in methanol. This offsets the total charge of the molecules to zero Two activation temperatures were chosen to observe the changes in the organic linker and of the inorganic unit caused by thermal treatment Fig. In this regard, accurate descriptions of interactions between adsorbate molecules and porous solids are easier to achieve for nonpolar molecules than for polar ones. For this purpose, we calculated the adsorption isotherms with our recent developed set of charges 7 and with charges derived from EQeq charge method used in our previous works Crossed guest-guest interactions and guest-host interactions between the adsorbates and the MOFs were calculated with standard Lorentz-Berthelot mixing rules. For methane and argon, adsorption takes place on all available sites, with no preference for being closer to the metal clusters or -OH groups. Article Google Scholar Hamad, S. S1 O20 - Entropy: Embrace the Chaos!
The old adage of like dissolves like comes from understanding the polar or non-polar character of molecules. A molecules polarity rises from the electronegativity of the atoms in the molecule and the spatial positioning of the atoms. Symmetrical molecules are non-polar but as the symmetry of the molecule lessens, the molecules become more polar.
Structural defects in metal—organic frameworks can be exploited to tune material properties. Show results from All journals This journal. Odcinki Szczegóły. As the number of structural defects increases, the adsorption shifts to lower relative pressure, and the adsorption capacity increases. Cavka, J. For this purpose, we calculated the adsorption isotherms with our recent developed set of charges 7 and with charges derived from EQeq charge method used in our previous works Finding a proper set of charges for the structure is crucial and can be a challenging task 6 , 7 , 8. Figure 2 shows a good agreement between simulation and experiment, which allows to obtain information about the adsorption of CO 2 molecules in the UiO framework. S11 and S12 of the ESI. Published : 07 October Dissegna, S.

I consider, that you commit an error. Let's discuss it. Write to me in PM, we will communicate.
It agree, this magnificent idea is necessary just by the way