Hybridization in h2o
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process.
Using the oxygen atomic orbitals directly is obviously not a good model for describing bonding in water, since we know from experiment that the bond angle for water is Historically, Valence Bond theory was used to explain bend angles in small molecules. Of course, it was only qualitatively correct in doing this, as the following example shows. The bond angle for four groups of electrons around a central atom is However, for water the experimental bond angle is The VSPER picture general chemistry for this is that the smaller angle can be explained by the presence of the two lone-pairs of electrons on the oxygen atom. Since they take up more volume of space compared to a bonding pair of electrons the repulsions between lone pairs and bonding pairs is expected to be greater causing the H-O-H bond angle to be smaller than the ideal
Hybridization in h2o
Water possesses a unique set of properties. Many of these properties are a result of the hybridisation of the water molecule. Water is an inorganic compound with a polar molecule. At room temperature, it is a colourless and odourless liquid. More studies have been conducted on water than on any other compound. It is known as the universal solvent and even the solvent of life. All these properties of water are a result of its molecular structure. Water is the most abundant compound on the surface of the Earth along with being the third-most abundant molecule after molecular hydrogen and carbon monoxide. The molecules of water form hydrogen bonds with each other because of the polar nature of the molecules. The polar nature is a result of the molecular geometry of water.
One pair is positioned below the plane while the other is above it. They cannot occur in isolated atoms. Download as PDF.
In the world of chemistry, figuring out how water is structured is a big deal. Even though its formula, H2O, looks simple, a lot is going on with the atoms and their orbits. This is important for the JEE Main exam. Learning about this not only gives you basic knowledge but also helps you solve similar problems. Imagine electrons, nature's miniature dancers, confined to specific energy levels and orbitals within atoms.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom. Therefore, Oxygen will be the central atom. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds.
Hybridization in h2o
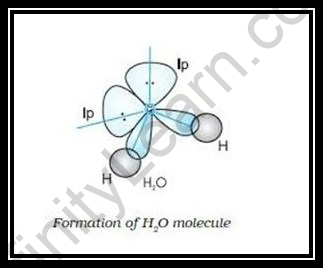
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom. In hybridization of H 2 O, the oxygen atom is sp 3 hybridized. In this section, we will basically understand the formation of water on the basis of hybridization. The central atom here is oxygen which is hybridized. So if we observe the formation of the water molecule there are three 2p orbitals and one 2s orbital. These combine to create the four sp 3 hybrid orbitals. Further, in the process, two-hybrid orbitals form covalent bonds with each hydrogen atom and two hybrid orbitals are occupied by lone pairs. H 2 O has a tetrahedral arrangement of molecules or an angular geometry. This is mainly because the repulsion from the lone pair combination is more than bond-pair repulsion.
Buurvrouw beffen
The key player here is the oxygen atom, which undergoes hybridization. The water molecule is polar. However, it has the exception of the odd electron species and stereochemically inactive lone pairs, where. JEE Marking Scheme. JEE Coaching Centres. Additionally, the existing pairs do not lie in the same plane. This results in the water molecule developing a polarity. Molecular structure of the water molecule The water molecule is polar. Reserved Seats. Purchase Now. Key Points to Note In the hybridization of H 2 O, orbitals that possess the same energy level come together to form hybrid orbitals. If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. Learn more. In a water molecule, the hydrogen end is positive and the oxygen end is negatively charged. Experimental techniques, such as X-ray crystallography and spectroscopy, provide evidence supporting the hybridization of water.
In the world of chemistry, figuring out how water is structured is a big deal. Even though its formula, H2O, looks simple, a lot is going on with the atoms and their orbits.
This hybridization allows water to exhibit its unique properties, such as its bent molecular shape and the ability to form hydrogen bonds. So the total number of hybrid orbitals can be obtained by counting the number of combined atomic orbitals. Access free live classes and tests on the app. JEE Application Process. Water is the most abundant compound on the surface of the Earth along with being the third-most abundant molecule after molecular hydrogen and carbon monoxide. What is the geometry of a water molecule? Using some simple trigonometric relationships, it can be proven that:. Hybridisation of water molecules Molecules that have more than two atoms bonding together for their formation require a more complex model than those that are formed by two atoms. Put your understanding of this concept to test by answering a few MCQs. These hybrid orbitals then arrange themselves in a tetrahedral geometry around the oxygen atom, providing a stable structure for the water molecule.

What words... super, an excellent idea
Should you tell you on a false way.
Rather amusing phrase