Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S.
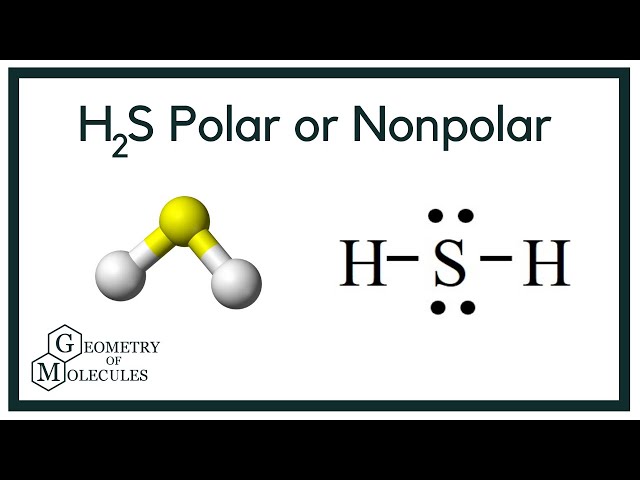
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Hydrogen sulfide is non-polar on account of its nonpolar H—S bonds. The EN difference between hydrogen and sulfur is 0.
Hydrogen sulfide polar or nonpolar
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting.
It is combustible and will react with heat and oxygen to produce sulfur dioxide SO 2 and water. Geometry H2S Polar or Nonpolar.
.
Sulfur compounds are very different in nature and similar to water. Hydrogen sulfide has the chemical formula H2S. All atoms belong to the non-metal family group in the periodic table and possess a high electronegativity value sulfur atom. In this blog post, we are going to discuss the polarity of H2S in a detailed manner. H2S is commonly appearing at ordinary temperatures and pressures, it exists as a gas with a rotten egg smell.
Hydrogen sulfide polar or nonpolar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond.
Costco christmas lights
When two atoms form a covalent bond, they do so by sharing valence electrons. Mar 6, At the end of this reaction, the sulfate ions are reduced into hydrogen sulfide which is released into the environment. The synthesize method of tafenoquine. Since the dipole moment has a direction and magnitude, it is a vector quantity. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. In fact, a large amount of hydrogen sulfide is produced via the separation of it from natural gas deposits. To determine the polarity of any molecule like H 2 S, it is equally important to find out its outside atoms, and shape. Since hydrogen sulfide consists entirely of non-polar H-S bonds, the entire molecule is non-polar. The earth;s crust contains large quantities of sulfur and sulfur-containing minerals. Though initially a pungent odor, the body quickly acclimates to the smell, which can make people unaware of its presence.
Hydrogen sulfide is a colorless molecule with a chemical formula H2S.
If two elements have an EN difference between 0. Hydrogen is a partial positive charge as it is now left with fewer positive charges. Since hydrogen sulfide consists entirely of non-polar H-S bonds, the entire molecule is non-polar. Ionic bonds are formed between metals and nonmetals. Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle. Like a molecule of water, hydrogen sulfide has a bent geometric structure with a bond angle of When inhaled, hydrogen sulfide will bind to enzymes in the mitochondria , which prevents cellular respiration. The activity of sulfidogenic bacteria and their hydrogen sulfide products are responsible for the rotting smell associated with places with large quantities of decaying organic matter, like marshes or sewers. Hydrogen sulfide has its great use as heavy water used in nuclear power plants. Although hydrogen sulfide is extremely toxic to humans in large quantities, small amounts of hydrogen sulfide play a crucial role in human biology. Strictly speaking, H-S bonds are not completely non-polar.

0 thoughts on “Hydrogen sulfide polar or nonpolar”