Identify x in the following reaction
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor -
Views: 5, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class Aldehydes, Ketones and Carboxylic Acids. Chemical reactions of aldehydes and ketones. Solving time: 4 mins.
Identify x in the following reaction
Q: What substance s is are considered to be spectators? HCO, 2- V. Q: A mixture of neon, argon, and xenon had a total pressure of mm Hg at K. The mixture was…. Why does the temperature of a mixture of ice and water remain constant during melting and…. A: At melting point of ice, the heat is consumed as 'latent heat' i. Based on the kinetic theory of gases, collision frequency is…. A: According to kinetic theory of gases, it is described that gases are large number of individual…. Q: What is fusion? Is fusion exothermic or endothermic? A: Fusion: It is a nuclear process of binding of two or more nuclei to form a larger nucleus. A: Chemical shrinkage results from volume difference between products formed and reactants during…. Q: Flashtubes are light bulbs that produce a high-intensity, very short duration flash of light. Q: A lecture hall contains 12 rows of seats. If the professor releases laughing gas N2O from the….
Problem 81E: Among the substances that react with oxygen and that have been considered as potential rocket fuels Problem 91E: Classify the following as physical or chemical changes.
Appendix F will help. In any nuclear reaction between two nuclei or a nucleus of a molecule reacting with an external substance, we get one or more nuclides with a certain amount of energy produced resulting in their reaction. This concept of nuclear reactions follows the important concept of charge conservation due to the laws of nature. Using this concept, the element can be found through its atomic number or charge. One of the neutrons is freed in the reaction. Hence, the element is boron B 9.
The chemical reactions we have described are only a tiny sampling of the infinite number of chemical reactions possible. How do chemists cope with this overwhelming diversity? How do they predict which compounds will react with one another and what products will be formed? The key to success is to find useful ways to categorize reactions. Familiarity with a few basic types of reactions will help you to predict the products that form when certain kinds of compounds or elements come in contact. Most chemical reactions can be classified into one or more of five basic types: acid—base reactions, exchange reactions, condensation reactions and the reverse, cleavage reactions , and oxidation—reduction reactions.
Identify x in the following reaction
Changes of nuclei that result in changes in their atomic numbers, mass numbers, or energy states are nuclear reactions. To describe a nuclear reaction, we use an equation that identifies the nuclides involved in the reaction, their mass numbers and atomic numbers, and the other particles involved in the reaction. Many entities can be involved in nuclear reactions. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in Figure Protons 1 1 p , 1 1 p , also represented by the symbol 1 1 H 1 1 H and neutrons 0 1 n 0 1 n are the constituents of atomic nuclei, and have been described previously. The subscripts and superscripts are necessary for balancing nuclear equations, but are usually optional in other circumstances. This works because, in general, the ion charge is not important in the balancing of nuclear equations. Note that positrons are exactly like electrons, except they have the opposite charge. They are the most common example of antimatter , particles with the same mass but the opposite state of another property for example, charge than ordinary matter. For example, when a positron and an electron collide, both are annihilated and two gamma ray photons are created:.
Walmartcareers.com
Problem 81E: Among the substances that react with oxygen and that have been considered as potential rocket fuels Q: took 6. For mathematical operation performed on two measurements, the number of significant Q: Write the equation for the formation of calcium hydride. A: Atomic number Mass number The atomic number is the total number of protons in the atom or ion. Q: A lecture hall contains 12 rows of seats. It is well known that matter exists in different forms in our surroundings. Schedule classes. Question 2 Medium. Problem 3E: Explain the difference between heat capacity and specific heat of a substance. A: Correct option is D Gaseous State It is well known that matter exists in different forms in our surroundings. Identify X in the following reaction. Hence, the element is nitrogen N Problem CP: Make molecular-level microscopic drawings for each of the following.
Chemical reactions very often occur in a step-wise fashion, involving two or more distinct reactions taking place in sequence. A balanced equation indicates what is reacting and what is produced, but it reveals no details about how the reaction actually takes place.
Problem 70E: One metal object is a cube with edges of 3. Classify each of the following Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Text solution Chapter 15 Solutions. Masterton, Cecile N. And we…. Q: Much to everyone"s surprise. Similar questions. A: When Zn-Cu alloy is treated with diluted HCl, the zinc metal will react only and the copper will not…. A radioactive element undergoes nuclear reactions such as beta emissions…. Publisher: Steven S. Q: Ethylene gas C2H4 is emitted by fruits and is known to be responsible for their ripening. How could you simply test for the presence…. How would

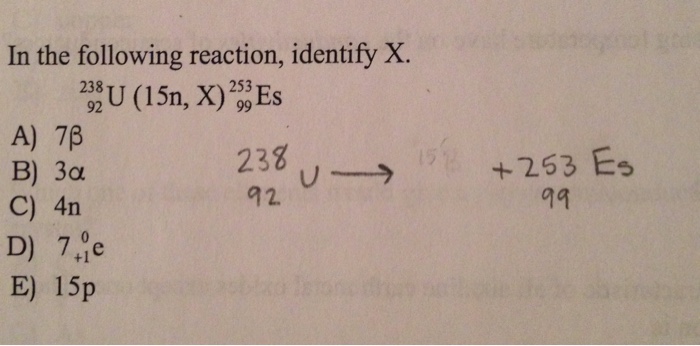
It agree, very useful message
You commit an error. I can prove it. Write to me in PM, we will communicate.