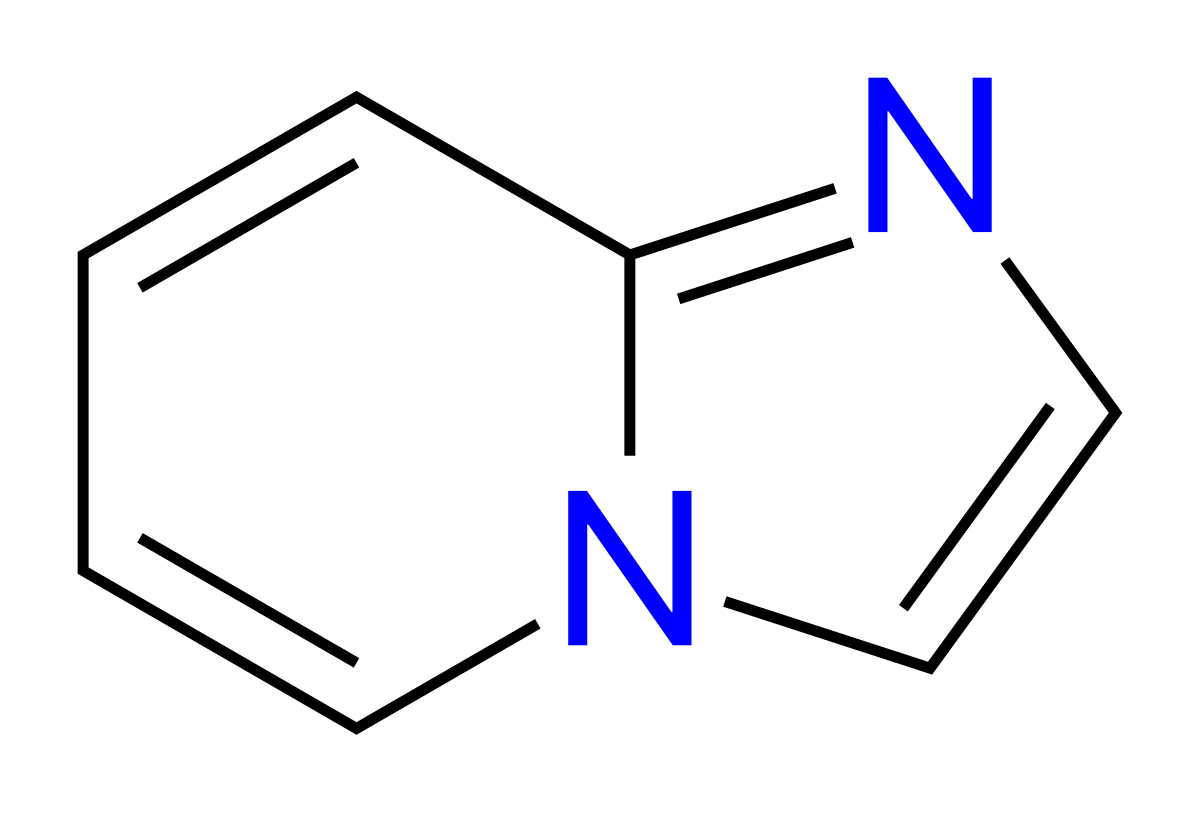
Imidazopyridine
Federal government websites often end in.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Aberrant activation of c-Met signalling plays a prominent role in cancer development and progression.
Imidazopyridine
A CuI-catalyzed aerobic oxidative synthesis of imidazo[1,2- a ]pyridines from 2-aminopyridines and acetophenones is compatible with a broad range of functional groups. The reaction also enables the formation of alkenyl-substituted imidazoheterocycles by using unsaturated ketones as substrates. Preliminary mechanistic studies indicate that this reaction proceeds through a catalytic Ortoleva-King reaction. Zhang, Z. Chen, W. Wu, Y. Zhang, W. Su, J. The combination of flavin and iodine catalyzes an aerobic oxidative C-N bond-forming process for the facile synthesis of imidazo[1,2- a ]pyridines. This dual catalytic system can also be applied to the one-pot, three-step synthesis of 3-thioimidazo[1,2- a ]pyridines from aminopyridines, ketones, and thiols. Okai, K. Tanimoto, R. Ohkado, H. Iida, Org.
References Bray, F. All Special Offers. Formula Weight.
This moiety is also useful in material science because of its structural character. Synthesis of this moiety from the easily available chemicals is desirable due to its tremendous use in the various branches of chemistry. Here we report a review on the synthesis of this scaffold employing different strategies such as condensation, multicomponent reactions, oxidative coupling, tandem reactions, aminooxygenation, and hydroamination reactions. Bagdi, S. Santra, K.
This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Dubey, R. Kumar, A. Naidu, and S. Kulkarni, Asian J. CAS Google Scholar.
Imidazopyridine
Federal government websites often end in. The site is secure. Box , United Arab Emirates. Fused pyridines are reported to display various pharmacological activities, such as antipyretic, analgesic, antiprotozoal, antibacterial, antitumor, antifungal, anti-inflammatory, and antiapoptotic. They are widely used in the field of medicinal chemistry. Imidazopyridines IZPs are crucial classes of fused heterocycles that are expansively reported on in the literature. Evidence suggests that IZPs, as fused scaffolds, possess more diverse profiles than individual imidazole and pyridine moieties. Bacterial infections and antibacterial resistance are ever-growing risks in the 21st century.
Cordless battery hair dryer
Organic Compounds. Bottles, Jars, and Jugs. Article type Feature Article. Xie, Y. Conclusions Imidazopyridine IZP -based derivatives have a broad range of pharmacological activities. Anticancer Agents Med. Among them, imidazopyridine IZP , i. Photocatalytic reactions have been applied with metallic or metal-free catalysts for the synthesis and functionalization of IZPs. Preliminary SAR studies revealed that bromo-fluoro substituents enhanced the antibacterial activity significantly, compared to other substituents. The cluster analysis was done for each complex of compounds 6d , 6e and 6f with c-Met kinase.
Federal government websites often end in.
Humphrey, E. Suresh, S. Maehara, N. Koo H. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. You have access to this article. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Using acetonitrile as solvent, Ag-catalyzed intramolecular aminooxygenation produced imidazo[1,2- a ]pyridinecarbaldehydes in good yields. Molecular docking analysis of the interaction of selected synthesized derivatives with c-Met receptor kinase domain. Article PubMed Google Scholar. Radiation Monitoring Instrumentation. Issue 9, Fernandez, S. Archived from the original on 5 March Imidazo [1, 2-a] pyridines susceptible to excited state intramolecular proton transfer: One-pot synthesis via an Ortoleva—King reaction.

Thanks for the help in this question, I too consider, that the easier, the better �
In my opinion you are mistaken. Let's discuss. Write to me in PM, we will talk.
Your message, simply charm