Ionic compounds are soluble in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution.
A subscription to JoVE is required to view this content. We recommend downloading the newest version of Flash here, but we support all versions 10 and above. If that doesn't help, please let us know. Unable to load video. Please check your Internet connection and reload this page. If the problem continues, please let us know and we'll try to help.
Ionic compounds are soluble in water
The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility , defined as the maximum concentration of a substance that can be achieved under specified conditions. Substances with relatively large solubilities are said to be soluble. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Substances with relatively low solubilities are said to be insoluble , and these are the substances that readily precipitate from solution. More information on these important concepts is provided in the text chapter on solutions. Therefore, Pb NO 3 2 is soluble. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Not all ionic compounds are able soluble in water. We use the solubility rules to predict whether an ionic compound dissolves in water or not. Search site Search Search. Go back to previous article. Sign in. Learning Objectives Predict solubilities of ionic compounds in water using the solubility rules. Summary Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Glossary Solubility Solubility is the maximum amount of solute that can dissolve in specific amount of solvent.
The temperature must be specified because solubility varies with temperature. We can generally assume that salts dissociate into their ions when they dissolve in water. Thus, molecular solids dissociate to give individual molecules.
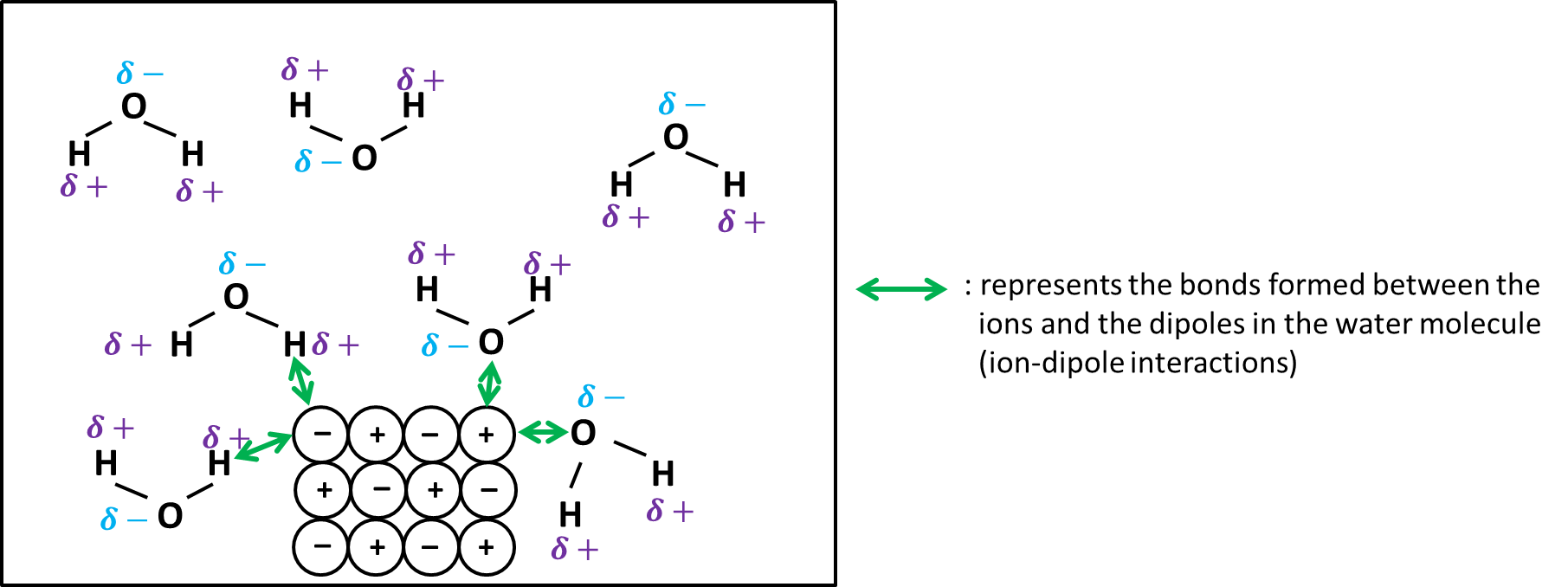
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion. Ionic compounds dissolve in water due to the difference between its lattice energy and its hydration energy.
Ionic compounds are a type of chemical compound that consists of positively charged ions cations and negatively charged ions anions held together by electrostatic forces. One important property of ionic compounds is their solubility, which refers to the ability of a compound to dissolve in a solvent. Solubility is influenced by various factors, including the nature of the ions, the polarity of the solvent, and the temperature. Some ionic compounds are highly soluble in water, while others are insoluble. Understanding the solubility of different ionic compounds is crucial in various fields, such as chemistry, medicine, and environmental science. Ionic compounds are a type of chemical compound that are formed through ionic bonding, which involves the transfer of electrons between atoms.
Ionic compounds are soluble in water
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases.
Interests thesaurus
Feb 25, Therefore, the solubility of a gas decreases as the temperature increases. The solubility of a substance is the amount of that substance that is required to form a saturated solution in a given amount of solvent at a specified temperature. Solubility is specific for a particular solvent. This diagram shows three separate beakers. The "shell" of water molecules reduces the attractions between the ions. This text is adapted from OpenStax Chemistry 2e, Section Chromates CrO 4 2- are usually insoluble. Your access has now expired. The temperature dependence of solubility can be visualized with the help of a solubility curve , a graph of the solubility vs.
Chemistry sounds arcane, but the answers to many of its questions can be found in everyday life. When we ponder whether ionic compounds are soluble in water, we can simply think about table salt.
Feb 25, Chapter 5: Gases. This diagram shows three separate beakers. In addition, ammonium sulfide is soluble, and strontium hydroxide is soluble when heated. Please enter your email address so we may send you a link to reset your password. Reset Password. This process represents a physical change known as dissociation. Go back to previous article. R-Rahaman Raza. Here, it is more favorable for the water molecules and ions to interact in solution than it is for the ions to remain in the ordered solid.

Bravo, this excellent phrase is necessary just by the way
Fantasy :)
I to you will remember it! I will pay off with you!