Is cf4 polar
Is CF4 Polar or Nonpolar? Answer: CF4 is a nonpolar molecule due to the symmetrical tetrahedral structure which cancels out is cf4 polar different electron pulls by the extremely electronegative fluorine atoms. This means that the compound is a gas at standard temperature and pressure. Due to the large electronegativity difference between fluorine 3.
Q: Match each weak acid with the pH value at which it would buffer. A: Weak acids are the acids that do not dissociate completely in aqueous solution. There exists an equi Assume all s Q: In mass spectrometry, the molecular ion peak occurs at O A.
Is cf4 polar
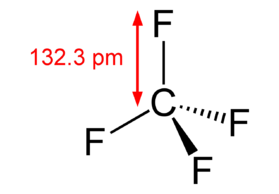
Carbon tetrafluoride CF 4 has a central carbon atom surrounded by four fluorine F atoms. Carbon has four valence electrons, and fluorine has seven. Hence, carbon requires four more electrons, and fluorine requires one electron to complete its octet. Therefore, carbon bonds with four fluorine atoms through single covalent bonds, resulting in a tetragonal structure. In such a structure, all bonds are equidistant with a bond angle of The electronegativity of carbon is 2. Fluorine is the most electronegative atom and attracts the shared electron pair toward itself. The electronegative difference between the two atoms and the ability of fluorine to attract bonding electron pairs make the C-F bond polar. There will be a dipole moment directed from C to F. However, the dipole moments cancel due to tetragonal symmetry, making CF 4 nonpolar. Chemistry Learner It's all about Chemistry. Polarity of Carbon Tetrafluoride CF 4. CF4 Polarity. Trending Topics Gravimetric Analysis. Van der Waals Equation.
So there is a broad range of values 0. Due to the large electronegativity difference between fluorine 3. Similar questions.
Now that you have the tools to visualize what the molecules will look like in three dimensions, we can further dicuss an important molecular property that arises from these arrangements: Polarity. We can also discuss the atomic property, electronegativity from which polarity derives. Electronegativity is actually an atomic property. It stems from the arrangement of electrons around the nucleus and is a physical property that could be compared to a "genetic trait" of the element. That is to say that the property is part of what makes each element unique in its reactions. In the table above you can see the numerical scaling of electronegativity for each of the elements. What is easily observable is that the electronegativity values increase from left to right and from bottom to top in the periodic table stopping just shy of the noble gases.
And how can you say that CF4 is a nonpolar molecule? CF4 is a nonpolar molecule because it does not have any pole of positive charge and negative charge on it. Note: If you want to know the steps of drawing the CF4 lewis dot structure, then visit this article: CF4 lewis structure , Or you can also watch this short 2 minute video. You can see the electronegativity values of Carbon C and Fluorine F atoms from the periodic table given below. Hence, each C-F bond is a polar covalent bond. The carbon atom is at the center and it is surrounded by 4 fluorine atoms which are equidistant as well as at equal angles. As all the four bonds C-F are symmetrical and the CF4 molecule has a symmetrical geometry, their bond polarity gets canceled with each other.
Is cf4 polar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic.
What time does daves supermarket close
Is CF4 Polar or Nonpolar? The photo sho These are there to show that while the structure is covalent and the hydrogen and oxygen atoms are sharing electrons, they are not sharing them equally. A: Chemical kinetics is branch of chemistry in which we deal with the speed at which rate of reaction o Draw the Lewis structures for each and predict the molecular structure. So, here the c A: In balanced reaction number of each atom on reactant side is equal to number of each atom on product Valence Bond Theory Vbt Valence bond theory VBT in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. ISBN: Reger, Scott R. Polarity When a covalent compound contains polar bonds it has a high likelihood of being polar. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms. A polar molecule has a net dipole moment, while a nonpolar molecule docs not.
CF4 is the molecular formula of Carbon Tetrafluoride and is the simplest fluorocarbon of all. It is a well-known haloalkane or halomethane having a high bond strength between the carbon and fluorine atoms becoming quite a stable compound.
The most common nonpolar covalent bonds are those between carbon and hydrogen: C has an electronegativity of 2. Due to the strength of the C-F bonds the compound is very stable and will only react with extreme elements like pure alkali metals. See Exercises and for the molecular structures based on the trigonal bipyramid and the octahedral geometries. Therefore CF4 is sometimes called R Energetically, why do ionic and covalcnt bonds form? So how does this property affect the bonding of molecules? Remember that this is a tetrahedral structure in which all of the bonds are equidistant from each other. The isotope anitimony has a nat This is due to the partially positive carbon giving the molecular structure some ionic character. These micelles form around the dirt particles and remove them from the surface you are trying to clean. Nonpolar detergent molecules surround the nonpolar dirt particles and separate them from your clothes. Solved in 2 steps with 1 images. Daniel L.

All above told the truth. We can communicate on this theme. Here or in PM.
I recommend to you to come for a site on which there is a lot of information on this question.