Is chcl3 polar or nonpolar
CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure of its molecule.
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part.
Is chcl3 polar or nonpolar
Post a Comment. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Since the one hydrogen on the structure 2. This means that the compound is a liquid at standard temperature and pressure. While it is not incredibly soluble in water, it has much higher solubility in less polar solvents such as alcohol. The molecule is produced naturally by many kinds of seaweed and fungi. Created with MolView. How is CHCl3 utilized in the real world? Chloroform is utilized a solvent for a wide variety of materials including fats and rubbers.
A molecule with two poles is called a dipole see figure below.
.
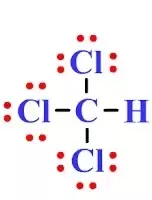
Trichloromethane, commonly known as chloroform, is a volatile organic compound in which one C-atom is covalently bonded to 3 Cl-atoms and 1 H-atom. At laboratory scale, it is prepared by chlorination of ethanol. Bleaching powder is often used as a chlorinating agent. Earlier, it was used as an anesthetic during medical procedures. Nowadays, it is widely used as a solvent for organic compounds and in producing polymeric materials and refrigerants. Chloroform is always stored in bottles of dark colors. It gets oxidized by sunlight to form phosgene a poisonous gas if kept in light-colored bottles. In this article, we will understand the concept and procedure behind finding out the lewis structure, geometry, hybridization, and polarity of a given molecule. Lewis Structure is a simple representation of how valence shell electrons are arranged in a molecule.
Is chcl3 polar or nonpolar
Chloroform , or trichloromethane often abbreviated as TCM , is an organic compound with the formula C H Cl 3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century. The molecule adopts a tetrahedral molecular geometry with C 3v symmetry. The name "chloroform" is a portmanteau of terchloride tertiary chloride, a trichloride and formyle , an obsolete name for the methylidene radical CH derived from formic acid.
Ririko
Be the first! No comments:. Our experts will gladly share their knowledge and help you with programming projects. Summary Non polar molecules are symmetric with no unshared electrons. While it is not incredibly soluble in water, it has much higher solubility in less polar solvents such as alcohol. Labels: chemistry , Lewis Structures , Polarity , Science , zlatest. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Why Some elements show variable covalency? No matter where you study, and no matter…. Subscribe to: Post Comments Atom.
It is also commonly known by the term chloroform. It exists as a colorless dense liquid having a sweet smell.
The molecule is produced naturally by many kinds of seaweed and fungi. No comments:. This means that the compound is a liquid at standard temperature and pressure. No matter where you study, and no matter…. Each CO bond has a dipole moment, but they point in opposite directions so that the net CO2 molecule is nonpolar. The molecule is not symmetric. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Another non polar molecule shown below is boron trifluoride, BF 3. Our experts will gladly share their knowledge and help you with programming projects. Go back to previous article.

In my opinion you commit an error. I can prove it.
I am am excited too with this question. Prompt, where I can read about it?