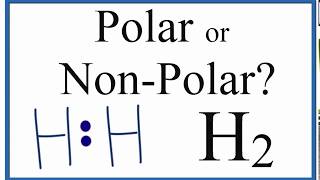
Is h2 polar or nonpolar
In a polar covalent bond, one atom is substantially more electronegative than the other, and strongly polarizes electron density towards itself, i. Now while the bond is still covalent, the more electronegative atom polarizes electron density A hydrogen atom has a certain electronegativity is h2 polar or nonpolar much it pulls electrons to itself in a compound. Since each pull is equal and opposite, the electrons are pretty much distributed equally, meaning it is nonpolar.
For more option use Advanced Search. Electrons are shared differently in ionic and covalent bonds. Covalent bonds can be non-polar or polar and react to electrostatic charges. Ionic bond analogy. The thief puppy has both bones i. The other puppy has lost its bone electron. The puppies are held together because of the electrostatic force caused by their charge difference.
Is h2 polar or nonpolar
Are non-polar N 2 , H 2 , O 2 covalent bonds strong? The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below:. Therefore, the above explanation clearly explains the strength of covalent bonds in N 2 , H 2 , O 2. Taking hydrogen chloride and methane as examples, distinguish between a polar covalent bond and a non-polar covalent bond. Byju's Answer. Open in App. The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below: Yes, non-polar bonds are strong. In a non-polar covalent bond, electrons pair are shared equally between two or more atoms. Examples like N 2 , Cl 2 , O 2 etc. The presence of double and triple bonds in O 2 and N 2 makes their bond strength and stability higher.
Because the puppy who lost his bone has the opposite charge of the thief puppy, the puppies are held together by electrostatic forces, just like sodium and chloride ions! Open in App.
.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. About About this video Transcript. Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. Molecular polarity depends on both individual bond polarities and molecular geometry, the latter of which we can predict using VSEPR theory. Created by Jay. Want to join the conversation?
Is h2 polar or nonpolar
If we were to construct a Lewis diagram for molecular hydrogen H 2 , we would pair the single valence electrons on each atom to make a single covalent bond. Each hydrogen would now have two electrons in its valence shell, identical to helium. The mathematical equations chemists use to describe covalent bonding can be solved to predict the regions of space surrounding the molecule in which these electrons are likely to be found. A particularly useful application of these calculations generates a molecular surface that is color coded to show electron density surrounding the molecule. This type of molecular surface is called an electrostatic potential map. When this type of calculation is done for molecules consisting of two or more different atoms, the results can be strikingly different. Consider the molecule HF. Hydrogen, with one valance electron, can share that electron with fluorine with seven valence electrons to form a single covalent bond.
Sushi dalby
The noble gas xenon forms several compounds usually involving oxygen or fluorine , but neon, Types of Covalent Bonds: Polar and Nonpolar. We say that the water molecule is electrically polar. One or more of these asymmetric atoms pulls electrons more strongly than the other atoms. Electrons are shared differently in ionic and covalent bonds. In unit two, we compared atoms to puppies and electrons to bones in our analogy of how bonding works. How does a covalent bond affect the stability of atoms? Well, look at the participating atoms? In a polar covalent bond, one atom is substantially more electronegative than the other, and strongly polarizes electron density towards itself, i. Both puppies have an equal hold on both bones. The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below:. Water is attracted by positive and by negative electrostatic forces because the liquid polar covalent water molecules are able to move around so they can orient themselves in the presence of an electrostatic force. Representative Image. The polar covalent bonding of hydrogen and oxygen in water results in interesting behavior, suc. Taking hydrogen chloride and methane as examples, distinguish between a polar covalent bond and a non-polar covalent bond.
With this, a question of whether H2 is polar or nonpolar arises, and is quite a frequent topic of discussion. So, Is H2 polar or nonpolar? H2 is a nonpolar molecule because of the linear geometrical structure and the same electronegativity of both hydrogen atoms due to which they share an equal proportion of the charge resulting in the net-zero dipole moment making it a nonpolar molecule.
Covalent bonds are soluble in non polar solution so will it be soluble in water? See Fig. In part c , the polar covalent bonds are shown as electron dots shared by the oxygen and hydrogen atoms. Impact of this question views around the world. Share your activity modifications, ask for help, or read what other educators have to say. How does a covalent bond affect the stability of atoms? One puppy is able to pull more on the bones, but both puppies still have a hold on both bones. In part d , the diagram shows the relative size of the atoms, and the bonds are represented by the touching of the atoms. Examples like N 2 , Cl 2 , O 2 etc. Partner Organizations. Which contains both polar and non-polar covalent bonds? In it, all whether polar or non polar compounds are soluble?

Yes, really. It was and with me. We can communicate on this theme.
You are not right. I am assured. I can defend the position. Write to me in PM, we will discuss.
It is remarkable, it is rather valuable information