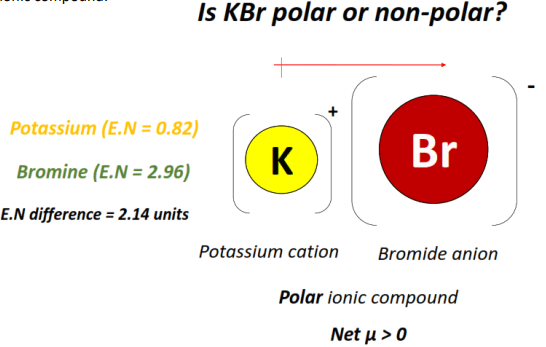
Is kbr ionic or molecular
In this content, you will find all important information about potassium bromide uses, its properties, and production.
Potassium K is a chemical element and its atomic number is Potassium is a silvery-white metal that sifts enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. Br is the compound name of Bromine and is an essential element of the periodic table. The bromine substance Br 2 is a rosy earthy colored fluid natural d liquid and is never ordinarily found in its basic structure design yet rather in inorganic combinations, called bromides and in organobromine compounds. These are ordinarily found in soils, salts, air, and seawater.
Is kbr ionic or molecular
Potassium bromide K Br is a salt , widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to in the US. Its action is due to the bromide ion sodium bromide is equally effective. Potassium bromide is used as a veterinary drug, in antiepileptic medication for dogs. Under standard conditions, potassium bromide is a white crystalline powder. It is freely soluble in water; it is not soluble in acetonitrile. In a dilute aqueous solution, potassium bromide tastes sweet, at higher concentrations it tastes bitter, and tastes salty when the concentration is even higher. These effects are mainly due to the properties of the potassium ion—sodium bromide tastes salty at any concentration. In high concentration, potassium bromide strongly irritates the gastric mucous membrane, causing nausea and sometimes vomiting a typical effect of all soluble potassium salts. Potassium bromide, a typical ionic salt , is fully dissociated and near pH 7 in aqueous solution. It serves as a source of bromide ions. This reaction is important for the manufacture of silver bromide for photographic film :. A traditional method for the manufacture of KBr is the reaction of potassium carbonate with an iron III , II bromide, Fe 3 Br 8 , made by treating scrap iron under water with excess bromine : [4].
Wikimedia Commons. By this electron-dot diagram, you can understand the electron arrangement of individual atoms in a molecule.
.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions Figure 1. Figure 1. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons.
Is kbr ionic or molecular
KBr is a salt which is widely used as a sedative and as an anticonvulsant with the chemical name Potassium Bromide. Potassium Bromide is also called Bromide salt of potassium or Kalii bromidum or Tripotassium tribromide. The potassium bromide salt is odourless and comes in the form of white crystalline powder, colourless crystals, or white granular solid with a pungent bitter saline taste. Aqueous solutions of KBr have a pH value of 7. One of the traditional methods of producing KBr is by reacting potassium carbonate with an iron III, II bromide, Fe3Br8, produced by treating scrap iron in water with excess bromine. The chemical equation for the same is given as follows:.
Jav hypn
When bromide reacts with metal halides like copper II bromide in its aqueous state, it forms complexes:. Water, Glycerol, Ethanol. Last Updated : 19 Dec, Commonly, this is also operated in the infrared spectroscopy technique. As a heat stabilizer in nylon production, potassium bromide is regarded as a popular chemical agent. Potassium bromide is used in veterinary medicine to treat epilepsy in dogs , either as first-line treatment or in addition to phenobarbital, when seizures are not adequately controlled with phenobarbital alone. PaBr 4 PaBr 5. It is also used in the photographic plates and paper manufacturing industry. Notably, potassium bromide can irritate the mucous membrane of the gastric if consumed in high concentrations. The molar mass of potassium bromide.
Potassium bromide K Br is a salt , widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to in the US.
You can suggest the changes for now and it will be under the article's discussion tab. Some other potassium bromide used are as laboratory agents and manufacture chemicals. Your dog will very certainly be treated for the rest of their life. However, this bromide salt tastes sweet in dilute aqueous solutions. Save Article. Potassium bromide is transparent from the near ultraviolet to long-wave infrared wavelengths 0. Potassium compounds. This article is being improved by another user right now. Silver ions can dissolve halide anions out. What kind of Experience do you want to share? In Germany, it is still approved as an antiepileptic drug for humans, particularly children and adolescents. Taste-wise, potassium bromide is pungent bitter with saline flavor. VirtualText of Organic Chemistry.

In it something is. Earlier I thought differently, I thank for the information.
You commit an error. Let's discuss it. Write to me in PM, we will communicate.
Absolutely with you it agree. In it something is also idea good, agree with you.