Is n2 polar
Are non-polar N 2H 2is n2 polar, O 2 covalent bonds strong? The explanation of the strength of covalent bonds in N 2H 2O 2 is explained below:. Therefore, the above explanation clearly explains the strength of covalent bonds in N 2H 2O 2. Taking hydrogen chloride and methane as is n2 polar, distinguish between a polar covalent bond and a non-polar covalent bond.
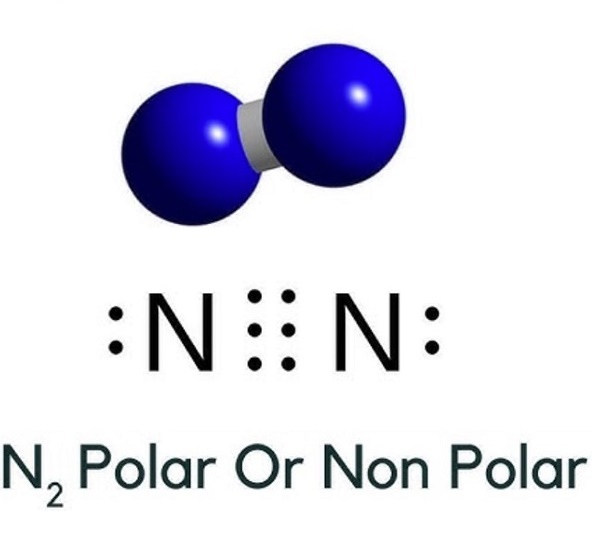
And how can you say that N2 is a nonpolar molecule? Here in N2 molecule, both the atoms are Nitrogen atoms. This value 0 is less than 0. Let me explain this in a short and simple way with 3D images. You can also watch this short 2 minute video. N2 is a nonpolar molecule because it does not have any pole of positive charge and negative charge on it. This is because both the atoms are identical i.
Is n2 polar
Have you ever done an experiment where you dip a flower in a cold substance and shatter it on a table like glass? That was liquid nitrogen. Even the foods you eat that can last for a long time was undoubtedly preserved with nitrogen gas. However, when we look at the chemical structure of N2, a common question a new chemist may have is if N2 is polar or nonpolar. So, is N2 polar or nonpolar? N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule. As a result, both atoms have equal electronegativity and share an equal proportion of charge and the overall molecule result in a net-zero dipole moment making it a nonpolar molecule. Nitrogen, or N2, is a very abundant and necessary chemical for biological life and industrial processes. Nitrogen is also plentiful in industrial chemistry, including fertilizers, dyes, nylon, and explosives. When atoms form bonds to create molecules, we can determine the level of polarity the molecule will exhibit.
MgF2 and NaCl are ionic. This value is less than 0. In a N2 molecule, there are two nitrogen atoms N.
Intermolecular forces are the forces that exist between molecules. Intermolecular forces of attraction are much weaker than intramolecular forces of attraction, but they are important because they determine the physical properties of molecules such as boiling point, melting point, density, and fusion and vaporisation enthalpies. Two atoms of the element bind to form N2, a colourless and odourless diatomic gas, at standard temperature and pressure. Nitrogen is found in all organisms, most notably in amino acids and thus proteins , nucleic acids DNA and RNA , and the energy transfer molecule adenosine triphosphate. The molecular geometry of N 2 is a linear structure, it is a nonpolar molecule. As a result, both atoms have equal electronegativity and share an equal proportion of charge, and the molecule as a whole has a net-zero dipole moment.
Nitrogen gas is used in many industrial and commercial applications, such as in the production of fertilizer and explosives. Nitrogen gas is the preferred choice for any medical application that requires gas to be confined within a container. Nitrogen gas is also used in inerting applications, such as when evacuating and purging piping prior to welding or brazing, for example. There are many uses for nitrogen gas beyond just medical use and industrial or commercial use that we will discuss in more detail throughout this article. Nitrogen gas, as we mentioned earlier, is the preferred choice for any confined-space application because it is safe and non-flammable. This means that the molecule has a dipole moment, with a positive end and a negative end. Water is an example of a polar molecule. This can also at times be referred to as being non-ionic or covalent in contrast to an ionic compound. The presence of water when cooking sugar creates a lower sugar concentration that decreases its ability to hold onto electrons without them being shared with other atoms in the solution. As such, sugar has an overall greater electrical charge which results in one end having a positive charge while the opposite end has a negative charge.
Is n2 polar
Dinitrogen or nitrogen gas is a nonpolar molecule having no electric dipole moment. Wondering how? Well, this detailed blog post will help you understand it. The molecule comprises two Nitrogen atoms making it a diatomic molecule.
Whirlpool 200l 3 star
When atoms form bonds to create molecules, we can determine the level of polarity the molecule will exhibit. The presence of double and triple bonds in O 2 and N 2 makes their bond strength and stability higher. It's ionic, not polar. Resources Leaderboard All Tags Unanswered. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations. Save my name, email, and website in this browser for the next time I comment. Is Barium hydroxide polar or nonpolar? Your result is as below. The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below: Yes, non-polar bonds are strong. And how can you say that N2 is a nonpolar molecule? This creates a dipole moment, which directs the electron density to the more electronegative molecule. Your email address will not be published.
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table , often called the pnictogens.
Created with MolView. When electrons are concentrated at one end of a molecule, it becomes slightly negative. There are two isomers of Ethenediol. November 28, Save my name, email, and website in this browser for the next time I comment. Related Posts. Nitrogen is a non polar gas. Nitrogen, like most elements on the periodic table, follows the octet rule, meaning it wants eight electrons in its outer shell. For example, Ammonia is a compound made of one nitrogen and three hydrogen molecules. Newer Post Older Post Home. The London dispersion force is a weak intermolecular force caused by electron motion in molecules, which creates temporary dipoles.

0 thoughts on “Is n2 polar”