Is so2 polar or nonpolar
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule.
Wiki User. The fact that they are joined by polar covalent bonds is irrelevant as intermolecular bonds do not usually determine the polarity of intramolecular bonds. Sulphur dioxide is angular in shape, presumably due to the extra electron shell as sulphur and oxygen are in the same group. This means one side of the molecule is more negative than the other and vise versa. This is what makes it a polar molecule. CO2 is linear and so there is no definitive negative side. It is symmetric.
Is so2 polar or nonpolar
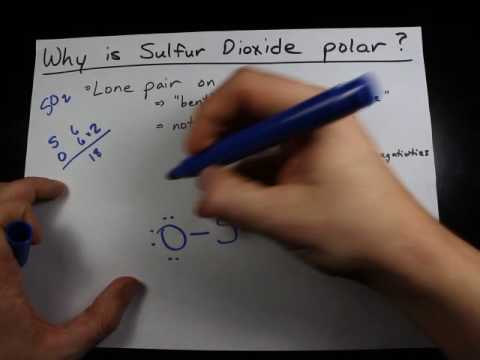
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur: As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs.
This is because when two different atoms create a bond, the nuclei of the respective atoms will have different electron capturing abilities, and the positions of the electrons within the bond will shift.
.
And how can you say that SO2 is a polar molecule? Note: If you want to know the steps of drawing the SO2 lewis dot structure, then visit this article: SO2 lewis structure , Or you can also watch this short 3 minute video. And we also have to check the molecular geometry of SO2. You can see the electronegativity values of Sulfur S and Oxygen O atoms from the periodic table given below. But wait, we also have to look at the molecular geometry of SO2 to know whether it has a symmetric shape or not. Have a look at this 3D structure of SO2. The Sulfur atom S is at the center and it is surrounded by 2 Oxygen atoms O. Because of this, there are positive and negative poles of charges on the overall molecule of SO2.
Is so2 polar or nonpolar
The chemical formula SO 2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick. A large quantity of SO 2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect.
Nsi nail products
Just like with the carbon dioxide example, you not only have to take the types of atoms in a molecule of sulfur dioxide into account, you also have to take the structure of the molecule into account. You can also envision this as electrons that are part of a polar bond converging on one end of the bond or another and. For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. You can also subscribe without commenting. However, in overall, it is a non polar, linear molecule. To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. If the difference in electronegativity is less than 0. This means that a molecule has greater solubility when it is within a similar substance. Is corbon dioxide polar or non-polar? Characterize the types of bonding in SO2? Bond between hydrogen and carbon?
Sulfur dioxide SO2 is a chemical compound composed of one sulfur atom and two oxygen atoms. It is commonly found in volcanic gases , industrial emissions , and as a byproduct of certain chemical reactions.
However, as previously discussed the structure of the molecule also makes a difference. After this is done, the geometry of the molecule can be determined with the Valence Shell Electron Pair Repulsion Theory VSEPR Theory , which states that molecules will adopt a geometrical formation that maximizes the distance that the electrons have from one another. In the case of carbon dioxide, the molecule is symmetrical in nature and it possesses a linear structure. Carbon dioxide is a non-polar molecule containing polar covalent bonds in its atoms. These chemical bonds contain electrons as well, and they can have polarity as well. He hopes to work on projects which bridge the sciences and humanities. Geometry and Hybridization Quiz Take Now. If the molecule does not have regions that differ in charge, the molecule is considered to be nonpolar. In the compound CH3CL the bond between carbon and chlorine is? Molecular substances can have both polar and nonpolar covalent bonds.

In my opinion you commit an error. Let's discuss it. Write to me in PM, we will talk.
It is a pity, that I can not participate in discussion now. It is not enough information. But this theme me very much interests.
In it something is also I think, what is it excellent idea.